Sepia Officinalis) Nothing but a Common Cheat?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Life Sciences, 2018; 6 (3):799-806 Life Sciences ISSN:2320-7817(P) | 2320-964X(O)
International Journal of Int. J. of Life Sciences, 2018; 6 (3):799-806 Life Sciences ISSN:2320-7817(p) | 2320-964X(o) International Peer Reviewed Open Access Refereed Journal Original Article Open Access Species diversity and basic biology of Cuttlefishes from Maharashtra waters, northwest coast of India Sundaram Sujit1 and Mane Sushant 2 1 Mumbai Research Centre of Central Marine Fisheries Research Institute, 2nd Floor, C.I.F.E old campus, Fisheries University road, Seven Bunglows, Versova, Mumbai - 400 061, Maharashtra, India. (Retd.) 2 Department of Zoology, Wilson College, Chowpaty, Mumbai-400 007, Maharashtra, India. Email- [email protected] Manuscript details: ABSTRACT Received :11.06.2018 Cuttlefish diversity was studied from Maharashtra waters during the period Accepted : 18.09.2018 January 2000 - December 2017. Eight species were identified and they are Published : 30.09.2018 Sepia pharaonis Ehrenberg, 1831, Sepia aculeata Orbigny, 1848, Sepia elliptica Hoyle, 1885, Sepiella inermis Orbigny, 1848, Sepia prashadi Editor: Dr. Arvind Chavhan Winckworth, 1936, Sepia (Doratosepion) kobiensis Hoyle, 1885, Sepia omani Cite this article as: Adam and Rees, 1966 and Euprymna berryi Sasaki, 1929. The estimated Sundaram Sujit and Mane Sushant annual catch of cuttlefishes by trawlers (all species combined) for the period (2018) Species diversity and basic 2000-2017 from New Ferry Wharf landing centre showed a cyclic trend and biology of Cuttlefishes from the landings ranged from 1360.4 t (2002) to a peak of 3,704.1 t (2012) and Maharashtra waters, northwest the corresponding catch rate ranged from 0.985 kg/hr (2002) to 1.599 coast of India, Int. J. -

Digital Cinematography Dedication
Digital Cinematography Dedication To my wife Anne With all my love Digital Cinematography Paul Wheeler BSC FBKS Focal Press An imprint of Elsevier Science Linacre House, Jordan Hill, Oxford OX2 8DP 225 Wildwood Avenue, Woburn, MA 01801-2041 First published 2001 Reprinted 2002 Copyright © 2001, Paul Wheeler. All rights reserved. The right of Paul Wheeler to be identified as the author of this work has been asserted in accordance with the Copyright, Designs and Patents Act 1988 All rights reserved. No part of this publication may be reproduced in any material form (including photocopying or storing in any medium by electronic means and whether or not transiently or incidentally to some other use of this publication) without the written permission of the copyright holder except in accordance with the provisions of the Copyright, Designs and Patents Act 1988 or under the terms of a licence issued by the Copyright Licensing Agency Ltd, 90 Tottenham Court Road, London, England W1T 4LP. Applications for the copyright holder’s written permission to reproduce any part of this publication should be addressed to the publishers British Library Cataloguing in Publication Data A catalogue record for this book is available from the British Library Library of Congress Cataloguing in Publication Data A catalogue record for this book is available from the Library of Congress ISBN 0 240 51614 1 Printed and bound in Great Britain Contents Preface xi About the author xiii Acknowledgements xv Introduction xvii PART ONE Digital Cinematography 1 1 Why digital -
OFFICIAL HANDBOOK of RULES and REGULATIONS 2020 | 68Th Edition
OFFICIAL HANDBOOK OF RULES AND REGULATIONS 2020 | 68th edition AMERICAN QUARTER HORSE An American Quarter Horse possesses acceptable pedigree, color and mark- ings, and has been issued a registration certificate by the American Quarter Horse Association. This horse has been bred and developed to have a kind and willing disposition, well-balanced conformation and agile speed. The American Quarter Horse is the world’s most versatile breed and is suited for a variety of purposes - from working cattle on ranches to international reining competition. There is an American Quarter Horse for every purpose. AQHA MISSION STATEMENT • To record and preserve the pedigrees of the American Quarter Horse, while maintaining the integrity of the breed and welfare of its horses. • To provide beneficial services for its members that enhance and encourage American Quarter Horse ownership and participation. • To develop diverse educational programs, material and curriculum that will position AQHA as the leading resource organization in the equine industry. • To generate growth of AQHA membership via the marketing, promo- tion, advertising and publicity of the American Quarter Horse. • To ensure the American Quarter Horse is treated humanely, with dignity, respect and compassion, at all times. FOREWORD The American Quarter Horse Association was organized in 1940 to collect, record and preserve the pedigrees of American Quarter Horses. AQHA also serves as an information center for its members and the general public on matters pertaining to shows, races and projects designed to improve the breed and aid the industry, including seeking beneficial legislation for its breeders and all horse owners. AQHA also works to promote horse owner- ship and to grow markets for American Quarter Horses. -

Along the Saudi Arabian Red Sea Coastline Thesis by Gordon Byron
Phylogenetic Diversity of Cephalopoda (Animalia:Mollusca) Along the Saudi Arabian Red Sea Coastline Thesis by Gordon Byron In Partial Fulfillment of the Requirements For the Degree of Master of Science King Abdullah University of Science and Technology Thuwal, Kingdom of Saudi Arabia © December, 2016 Gordon Byron All rights reserved 2 EXAMINATION COMMITTEE PAGE The thesis of Gordon Byron is approved by the examination committee. Committee Chairperson: Michael Berumen Committee Co-Chair: Christian Voolstra Committee Member: Timothy Ravasi 3 ABSTRACT Phylogenetic Diversity of Cephalopoda (Animalia:Mollusca) Along the Saudi Red Sea Coastline Gordon Byron Although the Red Sea presents a unique environment with high temperature and salinity, it remains an area that is understudied. This lack of information is reflected in many areas, one which is biodiversity. Despite increasing work on biodiversity throughout the Red Sea and an increase in Cephalopoda studies, Cephalopoda in the Red Sea remain underrepresented, which is especially pronounced in molecular analyses. Members of the class Cephalopoda are considered to be major contributors to coral reef ecosystems, serving as part of the food chain and exhibiting population increases due to targeted teleost fisheries and global climate change. In order to assess the biodiversity of Cephalopoda in the Saudi Arabian Red Sea, 87 specimens were collected from 25 reef locations between 17°N and 28°N latitude, as well as from the largest fish market in the Kingdom of Saudi Arabia. Taxonomic identification of specimens was determined using morphological comparisons with previously reported species in the Red Sea and the molecular barcoding region Cytochrome Oxidase I. 84 Red Sea sequences were compared with sequences from GenBank and analyzed using a complement of Neighbor- Joining, Maximum-Likelihood, and Bayesian inference trees. -

Striped Bodypainting Protects Against Horseflies
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Repository of the Academy's Library Striped bodypainting protects against horseflies royalsocietypublishing.org/journal/rsos Ga´bor Horva´th1,A´da´m Pereszle´nyi1,2, Susanne A˚kesson3 and Gyo¨rgy Kriska4,5 Research 1Environmental Optics Laboratory, Department of Biological Physics, ELTE Eo¨tvo¨s Lora´nd University, 1117 Budapest, Pa´zma´ny se´ta´ny 1, Hungary ´ Cite this article: Horva´th G, Pereszle´nyi A, 2Hungarian Natural History Museum, Department of Zoology, Bird Collection, 1083 Budapest, A˚kesson S, Kriska G. 2019 Striped bodypainting Ludovika te´r 2-6, Hungary 3 protects against horseflies. R. Soc. open sci. 6: Department of Biology, Centre for Animal Movement Research, Lund University, Ecology Building, 223 62 Lund, Sweden 181325. 4MTA Centre for Ecological Research, Danube Research Institute, 1113 Budapest, Karolina u´t http://dx.doi.org/10.1098/rsos.181325 29-31, Hungary 5Biological Institute, ELTE Eo¨tvo¨sLora´nd University, 1117 Budapest, Pa´zma´ny se´ta´ny 1, Hungary GH, 0000-0002-9008-2411; SA˚, 0000-0001-9039-2180 Received: 11 August 2018 Bodypainting is widespread in African, Australian and Papua Accepted: 11 December 2018 New Guinean indigenous communities. Many bodypaintings use white or bright yellow/grey/beige stripes on brown skin. Where the majority of people using bodypainting presently live, blood-sucking horseflies are abundant, and they frequently Subject Category: attack the naked brown regions of the human body surface with the risk of transmitting the pathogens of dangerous diseases. Biology (whole organism) Since horseflies are deterred by the black and white stripes of zebras, we hypothesized that white-striped paintings on dark Subject Areas: brown human bodies have a similar effect. -

ASFIS ISSCAAP Fish List February 2007 Sorted on Scientific Name
ASFIS ISSCAAP Fish List Sorted on Scientific Name February 2007 Scientific name English Name French name Spanish Name Code Abalistes stellaris (Bloch & Schneider 1801) Starry triggerfish AJS Abbottina rivularis (Basilewsky 1855) Chinese false gudgeon ABB Ablabys binotatus (Peters 1855) Redskinfish ABW Ablennes hians (Valenciennes 1846) Flat needlefish Orphie plate Agujón sable BAF Aborichthys elongatus Hora 1921 ABE Abralia andamanika Goodrich 1898 BLK Abralia veranyi (Rüppell 1844) Verany's enope squid Encornet de Verany Enoploluria de Verany BLJ Abraliopsis pfefferi (Verany 1837) Pfeffer's enope squid Encornet de Pfeffer Enoploluria de Pfeffer BJF Abramis brama (Linnaeus 1758) Freshwater bream Brème d'eau douce Brema común FBM Abramis spp Freshwater breams nei Brèmes d'eau douce nca Bremas nep FBR Abramites eques (Steindachner 1878) ABQ Abudefduf luridus (Cuvier 1830) Canary damsel AUU Abudefduf saxatilis (Linnaeus 1758) Sergeant-major ABU Abyssobrotula galatheae Nielsen 1977 OAG Abyssocottus elochini Taliev 1955 AEZ Abythites lepidogenys (Smith & Radcliffe 1913) AHD Acanella spp Branched bamboo coral KQL Acanthacaris caeca (A. Milne Edwards 1881) Atlantic deep-sea lobster Langoustine arganelle Cigala de fondo NTK Acanthacaris tenuimana Bate 1888 Prickly deep-sea lobster Langoustine spinuleuse Cigala raspa NHI Acanthalburnus microlepis (De Filippi 1861) Blackbrow bleak AHL Acanthaphritis barbata (Okamura & Kishida 1963) NHT Acantharchus pomotis (Baird 1855) Mud sunfish AKP Acanthaxius caespitosa (Squires 1979) Deepwater mud lobster Langouste -

Danielson, CT • 860-774-8588 Ending Aug
Serving Eastford, Pomfret & Woodstock Vol. III, No. 49 Complimentary to homes by request (860) 928-1818/e-mail: [email protected] ‘Life’s a voyage that’s homeward bound.’ FRIDAY, AUGUST 22, 2008 ‘Power of Woodstock Fair ready for Labor Day weekend the Press’ examines mid-1800s journalism NEXT PROGRAM WILL BE HELD SEPT. 17 BY MATT SANDERSON VILLAGER STAFF WRITER WOODSTOCK — Members of Roseland Cottage at Historic New ANNUAL EVENT RUNS FRIDAY THROUGH MONDAY England took a great task upon File photos themselves to not only preserve the BY MATT SANDERSON XtraMart Convenience Store, Above left: An aerial view of the 2007 Woodstock Fair. likeness of Henry Chandler VILLAGER STAFF WRITER Bowen’s accomplishments for which has several locations Above: Members of the Sandtasia sculptors of the Ocean State group creating a Woodstock, but also in the past year WOODSTOCK — As Labor Day throughout Connecticut, huge sandcastle for everyone attending the 2007 Woodstock Fair. to present his intrepid prowess to Weekend creeps closer, so does Massachusetts, Rhode Island and Below: A mother cow cleans her newborn baby at the 2007 Woodstock Fair. The the publication of his New York the 148th annual Woodstock Fair. New York. In addition, these tick- baby was born minutes before this photo was taken at the Barnyard Babies weekly periodical The Independent It runs this year from Friday, ets can be found at the Putnam Birthing Center. in the mid-1800s. The publication Aug. 29, through Monday, Sept. 1, Farmer’s Co-Op, Stoggy Hollow brought to the public eye some of at the Woodstock Fairgrounds Restaurant, Sweet Evalina’s the first American objective, jour- located on Route 171 in South Restaurant and the Thimble nalistic accounts leading up to the Woodstock. -

POPULATION DYNAMICS of the HOODED CUTTLEFISH Sepia Prashadi (WINCKWORTH, 1936) from the OMANI COASTAL WATERS of the ARABIAN SEA
7(1): 89-98 (2013) DOI: 10.3153/jfscom.2013010 Journal of FisheriesSciences.com E-ISSN 1307-234X © 2013 www.fisheriessciences.com RESEARCH ARTICLE ARAŞTIRMA MAKALESİ POPULATION DYNAMICS OF THE HOODED CUTTLEFISH Sepia prashadi (WINCKWORTH, 1936) FROM THE OMANI COASTAL WATERS OF THE ARABIAN SEA Sahar F. Mehanna∗, Dawood Al-Mamry Marine Science and Fisheries Centre, Muscat, OMAN Abstract: Basic population parameters of the hooded cuttlefish Sepia prashadi, in the Arabian Sea were described from samples collected during the demersal trawl survey of the Arabian Sea between September 2007 and August 2008. A total of 6869 S. prashadi with mantle lengths (ML) ranged from 3.4 to 21.2 cm were analyzed. Age and growth were studied using progression analysis model by applying Bhattacharya method. There were no significant differences in population parameters between sexes. The asymptotic ML was 24.13 cm, while the growth co- efficient K was 0.81/year and t0= -0.14 year. Mean total, natural and fishing mortalities were 3.66, 1.54 and 2.12 per year respectively. The exploitation ratio (E =0.58) suggests that the fishing pressure on S. prashadi in the Omani coastal waters is slightly high. Relative yield per recruit and relative biomass per recruit analysis showed that S. prashadi stock in the Arabian Sea is in its optimum situation as the current E is lower than that which gives the maximum Y’/R. For the management purpose and to reduce the risk due to the sampling bias, the current exploitation rate should be reduced by about 38% to achieve E0.5 as a target reference point and the present length at first capture should be raised to about 14 cm ML to conserve the first spawners of the stock. -

ISSN 0704-3716 Canadian Translation of Fisheries and Aquatic
ISSN 0704-3716 Canadian Translation of Fisheries and Aquatic Sciences No. 5377 Summaries of reports presented to the 4th All-Union Conference on Commercial Invertebrates Original title: Tezisy dokladov, IV Vsesoyuznaya konferentsiya po promyslovym bespozvonochnym. Chastu 1,2. 370 p. 1986. Publisher: All-Union Scientific Research Institute of Marine Fisheries and Oceanography (VNIRO). Moscow Original language: Russian Available from: Canada Institute for Scientific and Technical Information National Research Council Ottawa, Ontario, Canada KlA 0S2 1988 476 typescript pages 90 - 01551/ Secretary Secrétariat of State d'État MULTILINGUAL ERVICES DIVISION — DIVISION DES SERVICES MULTILINGUES TRANSLATION BUREAU BUREAU DES TRADUCTIONS LIBRARY IDENTIFICATION — FICHE SIGNALÉTIQUE Translated from - Traduction de Into - En Russian English Author - Auteur Title in English or French - Titre anglais ou français Summaries of reports presented to the 4th All—Union Conference on Commercial Invertebrates Title in foreign language (Transliterate foreign characters) Titre en langue étrangère (Transcrire en caractères romains) Tezisy dokladov,IV Vsesoyuznaya konferentsiya po promyslovym bespozvonochnym Reference in foreign language (Name of book or publication) in full, transliterate foreign characters. Référence en langue étrangère (Nom du livre ou publication), au complet, transcrire en caractères romains. same as title Reference in English or French - Référence en anglais ou français Publisher - Editeur Page Numbers in original DATE OF PUBLICATION Numéros des pages dans VNIRO DATE DE PUBLICATION l'original 1-173 Year Issue No. Volume Place of Publication Année Numéro Number of typed pages Lieu de publication Nombre de pages USSR dactylographiées 1986 474 Requesting Department Translation Bureau No. D F 0 3287260 Ministère-Client Notre dossier no Branch or Division I P B Translator (Initials) N. -
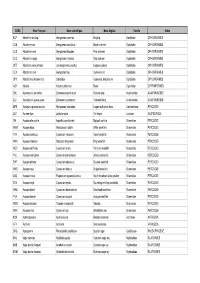
Nom Français
CODE Nom Français Nom scientifique Nom Anglais Famille Ordre KCP Abadèche du Cap Genypterus capensis Kingklip Ophidiidae OPHIDIIFORMES CUB Abadèche noir Genypterus maculatus Black cusk-eel Ophidiidae OPHIDIIFORMES CUS Abadèche rosé Genypterus blacodes Pink cusk-eel Ophidiidae OPHIDIIFORMES CUC Abadèche rouge Genypterus chilensis Red cusk-eel Ophidiidae OPHIDIIFORMES OFZ Abadèche sans jambes Lamprogrammus exutus Legless cuskeel Ophidiidae OPHIDIIFORMES CEX Abadèches nca Genypterus spp Cusk-eels nei Ophidiidae OPHIDIIFORMES OPH Abadèches, brotules nca Ophidiidae Cusk-eels, brotulas nei Ophidiidae OPHIDIIFORMES ALR Ablette Alburnus alburnus Bleak Cyprinidae CYPRINIFORMES ZML Acanthure à pierreries Zebrasoma gemmatum Spotted tang Acanthuridae ACANTHUROIDEI ZLV Acanthure à queue jaune Zebrasoma xanthurum Yellowtail tang Acanthuridae ACANTHUROIDEI MPS Achigan à grande bouche Micropterus salmoides Largemouth black bass Centrarchidae PERCOIDEI LQT Acmée râpe Lottia limatula File limpet Lottiidae GASTROPODA ISA Acoupa aile-courte Isopisthus parvipinnis Bigtooth corvina Sciaenidae PERCOIDEI WEW Acoupa blanc Atractoscion nobilis White weakfish Sciaenidae PERCOIDEI YNV Acoupa cambucu Cynoscion virescens Green weakfish Sciaenidae PERCOIDEI WKK Acoupa chasseur Macrodon ancylodon King weakfish Sciaenidae PERCOIDEI WEP Acoupa du Pérou Cynoscion analis Peruvian weakfish Sciaenidae PERCOIDEI YNJ Acoupa mongolare Cynoscion jamaicensis Jamaica weakfish Sciaenidae PERCOIDEI SWF Acoupa pintade Cynoscion nebulosus Spotted weakfish Sciaenidae PERCOIDEI WKS Acoupa -

Studies on Some Aspects Oflandings,Utilization and Export of Commercially Important Cephalopods
Studies on Some Aspects ofLandings, Utilization and Export of Commercially Important Cephalopods Thesis submitted to Cochin University of Science and Technology in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY By JOHN MOHAN School of Industrial Fisheries Cochin University of Science and Technology Cochin — 682 O16 April 2007 Certificate This is to certify that the thesis entitled “Studies on Some Aspects of Landings, Utilization and Export of Commercially Important Cephalopods” is a bonafide record of the research wcrk carried cut by Sri. John Mohan under my supervision and guiaance in the School of Industrial Fisheries, Cochin University of Science * id Technoivgy in partial fulfillment of the requirements for the degree of ' Q f Doctor of Philosophy of the Cochin University of SCi€m,€ ani Technology and no part thereof has been presented before _fL ' any other degree. Qa/xqéiwtfi AprilKochi -2007. 16 Prof. Supervising L ". M. Shahul Guide Hameed DECLARATION This is to certify that the thesis entitled “Studies on Some Aspects of Landings, Utilization and Export of Commercially Important Cephalopods” is an authentic record of the research work carried out by me under the supervision of Prof. (Dr). M. Shahul Hameed, Retd. Director, School of Industrial Fisheries, Cochin University of Science of Technology in partial-fulfillment of the requirements for the Ph.D degree of Cochin University of Science and Technology and no part of it has previously form.ed the basis for award of any degree in any University AprilKochi 2007 - 682016John Mohan \ ACKNOWLEDGEMENT I Wish to place on record my gratitude to Prof. -

Sepia Prabahari Neethiselvan and Venkataramani, 2002 Fig
110 FAO Species Catalogue for Fishery Purposes No. 4, Vol. 1 Sepia prabahari Neethiselvan and Venkataramani, 2002 Fig. 174 Sepia prabahari Neethiselvan and Venkataramani, 2002, Indian Journal of Marine Sciences, 31(1): 45 [type locality: southeast coast of India, Tuticorin, 08º47'N 78º9'E]. Frequent Synonyms: None. Misidentifications: None. FAO Names: En – Small striped cuttlefish; Fr – Petite seiche rayée; Sp – Sepia listada pequeña. male female Diagnostic Features: Male arms elongate, robust; arms I and IV elongate, whip-like (more pronounced in mature males); in females arms approximately subequal in length; arm formula in both sexes IV, I, III, II; arm suckers tetraserial. Hectocotylus present on left arm IV: 8 transverse rows of normal size suckers proximally, followed by 7 rows of modified suckers, then rest normal to arm tip. Suckers in 2 dorsal series greatly reduced, suckers in 2 ventral series normal in size on modified portion of arm; 2 dorsal and 2 ventral series displaced laterally, with fleshy ridge between. Club short, with 6 suckers in transverse rows; suckers all of similar minute size. Swimming keel terminates at posterior end of carpus. Dorsal protective membrane broader than ventral protective membrane. Dorsal and ventral protective membranes extend slightly beyond carpus, not joined at base of club. Cuttlebone elliptical; broader in females than males; rugose dorsally, with indistinct median and lateral ribs. Spine curved dorsally, without keels. Sulcus deep, broad, extends length of striated zone. Anterior striae are inverted V-shape. Inner male ventral view female hectocotylus cone limbs are narrow anteriorly, broaden posteriorly, cuttlebone then are raised into a thick, round ledge; outer cone Fig.