A Novel Vertebrate Eye Using Both Refractive and Reflective
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mono and Multifocals.Wps
Monovision and Multifocal Lenses THE GOAL: To provide you good functional vision without glasses at both distance and near most of the time. There are some demanding visual tasks in which monovision or multifocal (bifocal) lenses may not provide acceptable vision and additional glasses may be needed. MONOVISION Monovision interrupts the natural binocular (both eyes together) vision process by using one eye for distance viewing (usually the dominant eye) and the other eye for intermediate and near viewing. This is not how our visual systems were made, but monovision is an accepted modality and has been used for decades. Adapting to having one eye for distance and one for near takes time to adjust to , and is something that is very easy for some and very difficult for others. As the adaptation process proceeds vision becomes clear at all distances, as the brain suppresses the eye that is not being used. Initially you may feel that your distance vision is blurred by your near eye, as your near eye is focused for near and intermediate distances. This perception of distance blur should be reduced over time as the brain learns to suppress the near eye. Noticing a reduction in stereo vision, or depth perception is normal , as the eyes are not working, or teamed together. Noticing a mild reduction in night vision is normal with monovision. Everybody adapts differently , but is good to continue to try monovision for several consecutive days, or longer, to let the adaptation process begin. Monovision is a learned technique, and everybody’s timetable for adaptation is different. -

Suzi Fleiszig Researches the Cornea's Resistance To
UNIVERSITY OF CALIFORNIA VOL. 2, NO. 1 | FALL 2009 Berkeley Optometry magazine SUZI FLEISZIG RESEARCHES THE CORNEA’S RESISTANCE TO INFECTION ADVANCING EDUCATION AND RESEARCH AT BERKELEY OPTOMETRY EXECUTIVE EDITOR Lawrence Thal dean’s message MANAGING EDITOR Jennifer C. Martin EDITOR Barbara Gordon EXECUTIVE ART DIRECTOR Molly Sharp E ARE BLESSED TO LIVE IN “INTERESTING TIMES”! PHOTOGRAPHY WAt this writing, the U.S. economy is in the tank, the state of California Ken Huie is in the red, and I’m sure I don’t need to tell you that the budget situation at the University of California continues to be challenging. PRODUCTION MANAGER Molly Sharp In this environment, our priorities are to preserve and maintain our funda- mental missions—teaching, research, and public service. And I’m pleased to say WRITERS/CONTRIBUTORS that Berkeley Optometry continues to excel in all of these through the efforts of Suzi Fleiszig, Lu Chen, Cynthia Dizikes, our dedicated faculty and staff. In this spirit, it seems timely to celebrate some Lawrence Thal, Mika Moy, Pam of the highlights of the last year. Satjawatcharaphong, Joy Sarver Among other things, in this issue of Berkeley Optometry Magazine you will see DESIGN that we have a new faculty and student exchange agreement with Peking Medi- ContentWorks, Inc. cal University (PKU) Third Hospital; one of our students, Brian Snydsman, won the 2008 Essilor Optometry Superbowl at the annual meeting of the American Optometric Association and American Optometric Student Asso- Published by University of California, Berkeley, ciation; Professor Clifton Schor received the Charles F. Prentice Medal from School of Optometry Office of External the American Academy of Optometry at its annual meeting last October; and Relations and Professional Affairs Professor Suzanne Fleiszig (see cover) and David Evans won a grant from the Phone: 510-642-2622, Fax: 510-643-6583 Bill and Melinda Gates Foundation as part of the global Grand Challenges Send comments and letters to: Explorations initiative. -

Corneal Haze and Visual Outcome After Collagen Crosslinking for Keratoconus: a Comparison Between Total Epithelium Off and Partial Epithelial Removal Methods
Original Article Corneal haze and visual outcome after collagen crosslinking for keratoconus: A comparison between total epithelium off and partial epithelial removal methods Hasan Razmjoo, Behrooz Rahimi, Mona Kharraji1, Nima Koosha, Alireza Peyman Department of Ophthalmology, 1Medical Student Research Committee, Isfahan University of Medical Sciences, Isfahan, Iran Abstract Background: Keratoconus is an asymmetric, bilateral, progressive noninflammatory ectasia of the cornea that affects approximately 1 in 2000 of the general population. This may cause a significant negative impact on quality of life. Corneal collagen crosslinking (CXL) is one of the recently introduced methods that have been used to decrease the progression of keratoconus, in particular, as well as other corneal‑thinning processes. Materials and Methods: A total of 44 keratoconic eyes of 22 patients were enrolled in this randomized prospective study, after obtaining informed consent. In the first group, the corneal epithelium were totally removed and in the second group, the central 3 mm of epithelium was kept intact and partial removal was performed. After collagen crosslinking in both groups, comprehensive ophthalmologic examination was performed on all patients before and 6 months after the surgery. This article is registered at www.clinicaltrial.gov with registration number NCT01809977. Results: The difference between the two groups was not statistically significant regarding postoperative corneal haziness, refraction, and visual acuity (P > 0.05). However, comparison of pre‑ and postoperative parameters within each group revealed that total removal of the cornea has resulted in significant improvement of K‑max (P value: 0.01) and Q‑value (P value: 0.009); while eyes in partial removal group had better improvement of corrected vision (P value: 0.006). -

Light Propagation Through the Eye: Numerical Considerations and Applications to Presbylasik Surgery Analysis
Light propagation through the eye: numerical considerations and applications to presbylasik surgery analysis Julián Espinosa Tomás Optics Department, University of Alicante Group of Optics and Vision Science Carlos Illueca, PhD. David Mas, PhD. Jorge Pérez, PhD. Julián Espinosa, MSc. Vissum Corporation, Alicante Jorge Alió, PhD. Dolores Ortiz, PhD. Esperanza Sala, OD. Light patterns calculation inside the eye Transmittance evaluation of cornea Transmittance evaluation of crystalline lens Wave propagation (angular spectrum) up to the plane of interest. Applications to presbylasik surgery analysis Corneal transmittance evaluation: - Geometrical configuration 2 surfaces 1st surface: Corneal topography 2nd surface: Dubbelman 2003 =6.6 − 0.005 × 2 2 2 R2 age xy+ +(1 + Qz2 ) − 2 Rz 2 = 0 Q2 = −0.1 − 0.007 × age Corneal transmittance evaluation: - Optical path length Crystalline lens transmittance evaluation Dubbelman 2001 (Scheimpflug photography ) x2+ y 2 +(1 + Qz ) 2 − 2 Rz = 0 = − × =−6.4 + 0.03 × Rant 12.9 0.057 age ; Qant age =− + × =−6.0 + 0.07 × Rpost 6.2 0.012 age ; Qpost age Crystalline lens transmittance evaluation opznzzii≈+1( 21 ii −)cosδ 102 i +∆−( z i) cos ( δ 12 ii + δ ) Wave propagation Convergent patterns calculation λz exp −iπ m ɶ 2 × ()∆x 2 0 ()u∝ DFT −1 z µ 2 m∆ x m2 ()∆ x ×DFT u0 − i π 0 1 0 exp 2 N λ N z c ∆x2 z≤0 ≤ z Nyquist condition λN c zc = 20mm λ = 633nm Total eye ∆x= 6.7mm N = 3600 0 Φ =3 ∆x p()4 0 Wave propagation 2 ∆x0 Nyquist condition: Nλ ≥ zc N κ >1 N′ = λ′ = κλ Let us define , κ and 1 1 ∆ξ ∆ξ = ∆ξ ′ = = ′ δ x0 δx0 κ ′ ′ ∆x0 ∆ξ = N ∆x0 = ∆ x z ∆ξ′ ∆x= N ′ z Wave propagation Rectangle function κ=1 vs. -

Optical Modeling of Schematic Eyes and the Ophthalmic Applications
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Doctoral Dissertations Graduate School 8-2009 Optical Modeling of Schematic Eyes and the Ophthalmic Applications Bo Tan University of Tennessee - Knoxville Follow this and additional works at: https://trace.tennessee.edu/utk_graddiss Part of the Atomic, Molecular and Optical Physics Commons Recommended Citation Tan, Bo, "Optical Modeling of Schematic Eyes and the Ophthalmic Applications. " PhD diss., University of Tennessee, 2009. https://trace.tennessee.edu/utk_graddiss/63 This Dissertation is brought to you for free and open access by the Graduate School at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Doctoral Dissertations by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. To the Graduate Council: I am submitting herewith a dissertation written by Bo Tan entitled "Optical Modeling of Schematic Eyes and the Ophthalmic Applications." I have examined the final electronic copy of this dissertation for form and content and recommend that it be accepted in partial fulfillment of the requirements for the degree of Doctor of Philosophy, with a major in Physics. James W. L. Lewis, Major Professor We have read this dissertation and recommend its acceptance: Ying-Ling Chen, Horace W. Crater, Christian Parigger, Ming Wang Accepted for the Council: Carolyn R. Hodges Vice Provost and Dean of the Graduate School (Original signatures are on file with official studentecor r ds.) To the Graduate Council: I am submitting herewith a dissertation written by Bo Tan entitled ―Optical modeling of schematic eyes and the ophthalmic applications.‖ I have examined the final electronic copy of this dissertation for form and content and recommend that it be accepted in partial fulfillment of requirements for the degree of Doctor of Philosophy, with a major in Physics. -

Restoration of Visual Performance and Opsin Expression Within the Retina During Eye Regeneration in the Florida Fighting Conch (Strombus Alatus) Jamie M
University of South Carolina Scholar Commons Theses and Dissertations 2018 Restoration of visual performance and opsin expression within the retina during eye regeneration in the Florida fighting conch (Strombus alatus) Jamie M. Clark University of South Carolina Follow this and additional works at: https://scholarcommons.sc.edu/etd Part of the Marine Biology Commons Recommended Citation Clark, J. M.(2018). Restoration of visual performance and opsin expression within the retina during eye regeneration in the Florida fighting conch (Strombus alatus). (Master's thesis). Retrieved from https://scholarcommons.sc.edu/etd/4910 This Open Access Thesis is brought to you by Scholar Commons. It has been accepted for inclusion in Theses and Dissertations by an authorized administrator of Scholar Commons. For more information, please contact [email protected]. Restoration of visual performance and opsin expression within the retina during eye regeneration in the Florida fighting conch (Strombus alatus) by Jamie M. Clark Bachelor of Science University of South Carolina, 2017 _______________________________________________________ Submitted in Partial Fulfillment of the Requirements For the Degree of Master of Science in Marine Science College of Arts and Sciences University of South Carolina 2018 Accepted by: Daniel I. Speiser, Director of Thesis Tammi Richardson, Reader Jay Pinckney, Reader Cheryl L. Addy, Vice Provost and Dean of the Graduate School © Copyright by Jamie M. Clark, 2018 All Rights Reserved ii ACKNOWLEDGMENTS I would like to thank my advisor, Dr. Daniel Speiser, for his help in designing this study and for his revisions on this thesis. I would also like to thank Dr. Jay Pinckney for his assistance with the statistical analyses used. -

Corneal Ectasia Preferred Practice Pattern®
Corneal Ectasia PPP Secretary for Quality of Care Corneal Ectasia Preferred Timothy W. Olsen, MD Academy Staff Ali Al-Rajhi, PhD, MPH Andre Ambrus, MLIS Practice Pattern® Rachel Lastra Flora C. Lum, MD Doris Mizuiri Medical Editor: Susan Garratt Approved by: Board of Trustees September 22, 2018 © 2018 American Academy of Ophthalmology® All rights reserved AMERICAN ACADEMY OF OPHTHALMOLOGY and PREFERRED PRACTICE PATTERN are registered trademarks of the American Academy of Ophthalmology. All other trademarks are the property of their respective owners. Preferred Practice Pattern® guidelines are developed by the Academy’s H. Dunbar Hoskins Jr., MD Center for Quality Eye Care without any external financial support. Authors and reviewers of the guidelines are volunteers and do not receive any financial compensation for their contributions to the documents. The guidelines are externally reviewed by experts and stakeholders before publication. Correspondence: Ali A. Al-Rajhi, PhD, MPH, American Academy of Ophthalmology, P. O. Box 7424, San Francisco, CA 94120-7424. E-mail: [email protected]. 2 © 2018 by the American Academy of Ophthalmology https://doi.org/10.1016/j.ophtha.2018.10.021 P171 Published by Elsevier Inc. ISSN 0161-6420/18 Corneal Ectasia PPP Secretary for Quality of Care Corneal Ectasia Preferred Timothy W. Olsen, MD Academy Staff Ali Al-Rajhi, PhD, MPH Andre Ambrus, MLIS Practice Pattern® Rachel Lastra Flora C. Lum, MD Doris Mizuiri Medical Editor: Susan Garratt Approved by: Board of Trustees September 22, 2018 © 2018 American Academy of Ophthalmology® All rights reserved AMERICAN ACADEMY OF OPHTHALMOLOGY and PREFERRED PRACTICE PATTERN are registered trademarks of the American Academy of Ophthalmology. -

Ibn Al-‐Haytham the Man Who Discovered How We
IBN AL-HAYTHAM THE MAN WHO DISCOVERED HOW WE SEE IBN AL-HAYTHAM EDUCATIONAL WORKSHOPS 1 Ibn al-Haytham was a pioneering scientific thinker who made important contributions to the understanding of vision, optics and light. His methodology of investigation, in particular using experiment to verify theory, shows certain similarities to what later became known as the modern scientific method. 2 Themes and Learning Objectives The educational initiative "1001 Inventions and the World of Ibn Al-Haytham" celebrates the legacy of Ibn al-Haytham. The global initiative was launched by 1001 Inventions in partnership with UNESCO in 2015 in celebration of the United Nations International Year of Light. The initiative engaged audiences around the world with events at the UNESCO headquarters in Paris, United Nations in New York, the China Science Festival in Beijing, the Royal Society in London and in more than ten other cities around the world. Inspired by Ibn al-Haytham, exhibits, hands-on workshops, science demonstrations, films and learning materials take children on a fascinating journey into the past, sparking their interest in science while promoting integration and intercultural appreciation. This document includes a range of hands-on workshops and science demonstrations, with links to fantastic resources, for understanding the fundamental principles of light, optics and vision. The activities help engage young people to make, design and tinker while better understanding the significant contributions of Ibn al-Haytham to our understanding of both vision and light. Learning Objectives ü Inspire young people to study science, technology, engineering and maths (STEM) and pursue careers in science. ü Improve awareness of light, optics and vision through Ibn al-Haytham’s discoveries. -

Williams VR2011.Pdf
Vision Research 51 (2011) 1379–1396 Contents lists available at ScienceDirect Vision Research journal homepage: www.elsevier.com/locate/visres Review Imaging single cells in the living retina ⇑ David R. Williams Center for Visual Science, William G. Allyn Professor of Medical Optics, University of Rochester, Box 270270, Rochester, NY 14627-0270, United States article info abstract Article history: A quarter century ago, we were limited to a macroscopic view of the retina inside the living eye. Since Received 8 February 2011 then, new imaging technologies, including confocal scanning laser ophthalmoscopy, optical coherence Received in revised form 29 April 2011 tomography, and adaptive optics fundus imaging, transformed the eye into a microscope in which indi- Available online 10 May 2011 vidual cells can now be resolved noninvasively. These technologies have enabled a wide range of studies of the retina that were previously impossible. Keywords: Ó 2011 Elsevier Ltd. All rights reserved. Retinal imaging Confocal scanning laser ophthalmoscope Adaptive optics Optical coherence tomography Spatial resolution Ophthalmoscopy 1. Introduction Webster, 1886). Surprisingly, before the late 1980s there were few improvements in optical resolution since Helmholtz’s land- The ability to look inside the living human eye is central to our mark invention. A rare example is the incorporation of the elec- understanding of how the normal eye works and the diseased eye tronic flash, invented 30 years earlier by Edgerton, that allowed fails. This article focuses on technological advances in the spatial exposures brief enough to avoid eye motion blur (Ogle & Rucker, resolution of retinal imaging during the last quarter century and 1953). -

Newsletter of the Oceanic Engineering Society
Newsletter of the Oceanic Engineering Society MARCH 2016, Volume 5, Number 1 www.ieeeoes.org (USPS 025-095) ISSN 2164-8042 The OES BEACON is published four times a year as a benefit to the membership of the IEEE Ocean Engineering Society. The OES Beacon is printed and distributed from IEEE headquarters in New York City, New York, USA. Editor-in-Chief: Associate Editors: Contributing Editors: Harumi Sugimatsu – [email protected]. Masakazu Arima Australia – Mal Heron ac.jp Kenichi Asakawa France – Philippe Courmontague Co-Editor-in-Chief: Katsuyoshi Kawaguchi India – M. A Atmanand Robert L. Wernli – [email protected] Toshihiro Maki Japan – Blair Thornton IEEE OES VP – Professional Activities Takumi Matsuda Korea – Son Cheol Yu Associate Editor-in-Chief Hisashi Shiba Singapore – Venu Pallayil Kevin Hardy – [email protected] Blair Thornton Taiwan – Jenhwa Guo VP-PA Committee Publication Copy-Due schedule: 2nd Qtr: June 2016: May 14 3rd Qtr: September 2016: August14 Members are encouraged to submit copy highlighting 1) Chapter Events, 2) People & Company News, 3) Student & Young Professional News, 4) Technology Updates, or 5) other material of broad interest to the OES. Please send to Beacon Editor-in-Chief, Harumi Sugimatsu <[email protected] tokyo.ac.jp>. Word format, 1-1/2 space; Photos (always encouraged): jpg, 300 dpi preferred. Material becomes property of IEEE-OES. Please send e-mail or physical address corrections or updates to the EIC. IEEE OCEANIC ENGINEERING SOCIETY EXECUTIVE COMMITTEE President Vice President Treasurer RENÉ M. GARELLO Conference Development WILLIAM J. KIRKWOOD [email protected] ALBERT (SANDY) J. WILLIAMS III Monterey Bay Aquarium Research Institute Woods Hole Oceanographic Institute [email protected] Vice President [email protected] Technical Activities Journal Editor-in Chief KENNETH G. -

Correlation Analysis and Multiple Regression Formulas of Refractive Errors and Ocular Components
Correlation of refractive errors and ocular components ·Brief Report· Correlation analysis and multiple regression formulas of refractive errors and ocular components Chao-Kai Chang1, Jui-Teng Lin2,3, Yong Zhang4 1Nobel Eye Institute, Taipei 100, Taiwan, China corneal power and lens power[1-5]. However, the association 2New Vision Inc., Taipei 103, Taiwan, China between refractive errors and ocular biometrics such as 3Gong-Rui Medical Technology, Xiamen 361000, Fujian corneal power, central corneal thickness, anterior chamber Province, China depth (ACD), and lens thickness are inconclusive[6-13]. The 4Department of Ophthalmology, Shandong Provincial Hospital, recent study of Hashemi et al[14] for individuals aged 40-64y, Shandong University, Jinan 250021, Shandong Province, China showed that corneal power and axial length make the greatest Co-first authors: Chao-Kai Chang and Jui-Teng Lin contribution to spherical equivalent in high hyperopia and high Correspondence to: Jui-Teng Lin. New Vision Inc., Taipei myopia. Moreover, anterior segment biometric components 103, Taiwan, China. [email protected]; Yong Zhang. show stronger correlation in hyperopia than in myopia. Department of Ophthalmology, Shandong Provincial Hospital, They also reported that lower levels of refractive errors are Jinan 250021, Shandong Province, China. yzhangmd@ attributed to an imbalance among the components of ocular hotmail.com biometrics, whereas at higher levels, ACD plays a greater role. Received: 2018-06-19 Accepted: 2018-09-10 Due to the variation and correlations of biometric components during growth, the refractive error of the eye is governed Abstract by the multifactorial condition involving corneal and lens ● The multiple regression formulas and correlation of power, ACD and vitreous chamber depth (VCD) of the eye. -
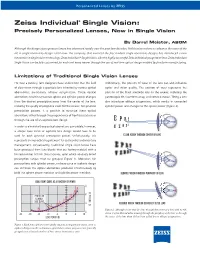
Zeiss Individual® Single Vision: Precisely Personalized Lenses, Now in Single Vision
Personalized Lenses by ZEISS Zeiss Individual® Single Vision: Precisely Personalized Lenses, Now in Single Vision By Darryl Meister, ABOM Although the design of progressive lenses has advanced rapidly over the past few decades, little has been done to advance the state of the art in single vision lens design. Until now. The company that invented the first modern single vision lens designs has introduced a new innovation in single vision technology: Zeiss Individual® Single Vision. Like the highly successful Zeiss Individual progressive lens, Zeiss Individual Single Vision can be fully customized for each and every wearer through the use of real-time optical design enabled by free-form manufacturing. Limitations of Traditional Single Vision Lenses For over a century, lens designers have understood that the field Additionally, the position of wear of the lens can also influence of clear vision through a spectacle lens is limited by various optical optics and vision quality. The position of wear represents the aberrations, particularly oblique astigmatism. These optical position of the fitted spectacle lens on the wearer, including the aberrations result in unwanted sphere and cylinder power changes pantoscopic tilt, face-form wrap, and vertex distance. Tilting a lens from the desired prescription away from the center of the lens, also introduces oblique astigmatism, which results in unwanted reducing the quality of peripheral vision for the wearer. For spherical cylinder power and changes to the sphere power (Figure 2). prescription powers, it is possible to minimize these optical aberrations either through the proper choice of front (base) curve or through the use of an aspheric lens design.