Interpretation of Ground and Surface Water Quality
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Justification of the Project in Forest Area 132Kv Nirjuli-Gohpur Transmission Line
JUSTIFICATION OF THE PROJECT IN FOREST AREA 132KV NIRJULI-GOHPUR TRANSMISSION LINE Ministry of Civil Aviation, Govt of India has accorded approval for setting up a Domestic Greenfield Airport at Hollongi, Itanagar, Arunachal Pradesh for “Public Use” category vide No. AV.204018/25/2015-AD Dated: 18-01-2019. Subsequently, Hon'ble Prime Minister of India, Sh. Narendra Modi, has laid the foundation stone for the project on 09-Feb-2019. Government of Arunachal Pradesh has handed over 667 acres to Airport Authority of India through District Administration, Papum Pare, Arunachal Pradesh as a follow up action for initiating the works related to the project. The land handed over to AAI by District Administration of Papum Pare, Arunachal Pradesh has an 132KV inter-state transmission line, maintained by Power Grid Corporation of India Ltd. The said line is running through the allocated land of the airport, hence it needs to be shifted/diverted to facilitate the construction of Greenfield Airport. It is to mentioned that the 132KV inter-State transmission line is connected to 132/33KV Gohpur Sub-Station of AEGCL from POWERGRID Nirjuli Sub-Station and caters power requirement of the adjoining areas of Gohpur. The line corridor is interfering the Aircraft's approach & takeoff funnel as well as the boundary of the Hollongi Airport. Hence the shifting of the said high tension line is necessary to facilitate the landing approach of the aircraft and to facilitate the construction of the Greenfield airport at Hollongi. A pictorial view of the airstrip runway and the approach & takeoff funnel is shown in the attached map below for ready reference. -

LIST of ACCEPTED CANDIDATES APPLIED for the POST of GD. IV of AMALGAMATED ESTABLISHMENT of DEPUTY COMMISSIONER's, LAKHIMPUR
LIST OF ACCEPTED CANDIDATES APPLIED FOR THE POST OF GD. IV OF AMALGAMATED ESTABLISHMENT OF DEPUTY COMMISSIONER's, LAKHIMPUR Date of form Sl Post Registration No Candidate Name Father's Name Present Address Mobile No Date of Birth Submission 1 Grade IV 101321 RATUL BORAH NAREN BORAH VILL:-BORPATHAR NO-1,NARAYANPUR,GOSAIBARI,LAKHIMPUR,Assam,787033 6000682491 30-09-1978 18-11-2020 2 Grade IV 101739 YASHMINA HUSSAIN MUZIBUL HUSSAIN WARD NO-14, TOWN BANTOW,NORTH LAKHIMPUR,KHELMATI,LAKHIMPUR,ASSAM,787031 6002014868 08-07-1997 01-12-2020 3 Grade IV 102050 RAHUL LAMA BIKASH LAMA 191,VILL NO 2 DOLABARI,KALIABHOMORA,SONITPUR,ASSAM,784001 9678122171 01-10-1999 26-11-2020 4 Grade IV 102187 NIRUPAM NATH NIDHU BHUSAN NATH 98,MONTALI,MAHISHASAN,KARIMGANJ,ASSAM,788781 9854532604 03-01-2000 29-11-2020 5 Grade IV 102253 LAKHYA JYOTI HAZARIKA JATIN HAZARIKA NH-15,BRAHMAJAN,BRAHMAJAN,BISWANATH,ASSAM,784172 8638045134 26-10-1991 06-12-2020 6 Grade IV 102458 NABAJIT SAIKIA LATE CENIRAM SAIKIA PANIGAON,PANIGAON,PANIGAON,LAKHIMPUR,ASSAM,787052 9127451770 31-12-1994 07-12-2020 7 Grade IV 102516 BABY MISSONG TANKESWAR MISSONG KAITONG,KAITONG ,KAITONG,DHEMAJI,ASSAM,787058 6001247428 04-10-2001 05-12-2020 8 Grade IV 103091 MADHYA MONI SAIKIA BOLURAM SAIKIA Near Gosaipukhuri Namghor,Gosaipukhuri,Adi alengi,Lakhimpur,Assam,787054 8011440485 01-01-1987 07-12-2020 9 Grade IV 103220 JAHAN IDRISH AHMED MUKSHED ALI HAZARIKA K B ROAD,KHUTAKATIA,JAPISAJIA,LAKHIMPUR,ASSAM,787031 7002409259 01-01-1988 01-12-2020 10 Grade IV 103270 NIHARIKA KALITA ARABINDA KALITA 006,GUWAHATI,KAHILIPARA,KAMRUP -

Class-8 New 2020.CDR
Class - VIII AGRICULTURE OF ASSAM Agriculture forms the backbone of the economy of Assam. About 65 % of the total working force is engaged in agriculture and allied activities. It is observed that about half of the total income of the state of Assam comes from the agricultural sector. Fig 2.1: Pictures showing agricultural practices in Assam MAIN FEATURES OF AGRICULTURE Assam has a mere 2.4 % of the land area of India, yet supports more than 2.6 % of the population of India. The physical features including soil, rainfall and temperature in Assam in general are suitable for cultivation of paddy crops which occupies 65 % of the total cropped area. The other crops are wheat, pulses and oil seeds. Major cash crops are tea, jute, sugarcane, mesta and horticulture crops. Some of the crops like rice, wheat, oil seeds, tea , fruits etc provide raw material for some local industries such as rice milling, flour milling, oil pressing, tea manufacturing, jute industry and fruit preservation and canning industries.. Thus agriculture provides livelihood to a large population of Assam. AGRICULTURE AND LAND USE For the purpose of land utilization, the areas of Assam are divided under ten headings namely forest, land put to non-agricultural uses, barren and uncultivable land, permanent pastures and other grazing land, cultivable waste land, current fallow, other than current fallow net sown area and area sown more than once. 72 Fig 2.2: Major crops and their distribution The state is delineated into six broad agro-climatic regions namely upper north bank Brahmaputra valley, upper south bank Brahmaputra valley, Central Assam valley, Lower Assam valley, Barak plain and the hilly region. -

Arunachal Pradesh Information Commission, Itanagar
ARUNACHAL PRADESH INFORMATION COMMISSION, ITANAGAR ANNUAL REPORT 2016 - 2017 1 The real Swaraj will come not by the acquisition of authority by a few, but by the acquisition of capacity by all to resist authority when abused. - MAHATMA GANDHI “Laws are not masters but servants, and he rules them who obey them”. -HENRY WARD BEECHER “Democracy requires an informed citizenry and transparency of information which are vital to its functioning and also to contain corruption and to hold Government and their instrumentalities accountable to the governed” ( Preamble, RTI Act 2005 ) 2 ACKNOWLEDGMENT This 11th & 12th Annual Reports of Arunachal Pradesh Information Commission 2016 - 2017 has been prepared in one volume. The data for preparation of this report are collected from Government Departments of the State. According to Information provided by the departments, the total number of Public Authorities in the State is 30 and the number of Public Information Officers is more than 310. The Right to Information Act, 2005 is a landmark legislation that has transformed the relationship between the citizen and the State. This legislation has been created for every citizen, to hold the instrumentalities of Governance accountable on a day to day basis. The legislation perceives the common man as an active participant in the process of nation building by conferring on him a right to participate in the process through the implementation of the Right to Information Act. It is more than a decade Since the RTI Act has been in operation in the State. The State Information Officers and Appellate Authorities are quasi judicial functionaries under the RTI Act with distinctive powers and duties and they constitute the cutting edge of this “Practical regime of information”, as envisaged in the preamble of the Right to Information Act. -
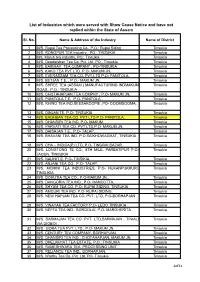
List of Industries Which Were Served with Show Cause Notice and Have Not Replied Within the State of Assam
List of Industries which were served with Show Cause Notice and have not replied within the State of Assam Sl. No. Name & Address of the Industry Name of District 1 M/S. Rupai Tea Processing Co., P.O.: Rupai Siding Tinsukia 2 M/S. RONGPUR TEA Industry., PO.: TINSUKIA Tinsukia 3 M/s. Maruti tea industry, PO.: Tinsukia Tinsukia 4 M/S. Deodarshan Tea Co. Pvt. Ltd ,PO.: Tinsukia Tinsukia 5 M/S. BAIBHAV TEA COMPANY , PO-TINSUKIA Tinsukia 6 M/S. KAKO TEA PVT LTD. P.O- MAKUM JN, Tinsukia 7 M/S. EVERASSAM TEA CO. PVT.LTD P.O- PANITOLA, Tinsukia 8 M/S. BETJAN T.E. , P.O.- MAKUM JN, Tinsukia 9 M/S. SHREE TEA (ASSAM ) MANUFACTURING INDMAKUM Tinsukia ROAD., P.O.: TINSUKIA 10 M/S. CHOTAHAPJAN TEA COMPNY , P.O- MAKUM JN, Tinsukia 11 M/S. PANITOLA T.E. ,P.O- PANITOLA , Tinsukia 12 M/S. RHINO TEA IND.BEESAKOOPIE ,PO- DOOMDOOMA, Tinsukia 13 M/S. DINJAN TE, P.O- TINSUKIA Tinsukia 14 M/S. BAGHBAN TEA CO. PVT LTD P.O- PANITOLA, Tinsukia 15 M/S. DHANSIRI TEA IND. P.O- MAKUM, Tinsukia 16 M/S. PARVATI TEA CO. PVT LTD,P.O- MAKUM JN, Tinsukia 17 M/S. DAISAJAN T.E., P.O- TALAP, Tinsukia 18 M/S. BHAVANI TEA IND. P.O.SAIKHOWAGHAT, TINSUKIA Tinsukia 19 M/S. CHA – INDICA(P) LTD, P.O- TINGRAI BAZAR, Tinsukia 20 M/S. LONGTONG TE CO., 8TH MILE, PARBATIPUR P.O- Tinsukia JAGUN, TINSUKIA 21 M/S. NALINIT.E. P.O- TINSIKIA, Tinsukia 22 M/S. -

Chapter-1 Population
CHAPTER-1 POPULATION Table 1.0- Population of Assam, at a Glance (as per 2011 census) Geographical Area (in Sq Km) 78438 No. of Districts (including six newly created districts) 33* No. of C.D. Block 219 No. of Sub-division (including 24 newly created sub-division) 80** No. of Circles 184 No. of Towns 214 No. of Statutory Towns 88 No. of Census Towns 126 No. of Villages 26395 No. of Households Total 6406471 Percentage of Rural Households 84.6 Population Persons 31205576 Percentage of Rural Population 85.9 Decadal growth rate (%) during 2001 over 2011 17.07 Annual Exponential Growth Rate 1.58 Sex Ratio (per `000 male) 958 Density of Population per Sq Km 398 Child(0-6yr) Population Persons 4638130 Child (0-6yr) Sex Ratio (per `000 male) 962 Literacy Rate (%) Total 72.19 Male 77.85 Female 66.27 Rural 69.34 Urban 88.47 * including newly created districts namely Biswanath, Charaideo, Hojai, South Salmara/Mankachar, West Karbi Anglong & Majuli. -1- Table1.1- Population Trends in Assam during 1901 -2011 Population Rural Population Urban Population Density of Year Growth Sex Population Decadal Decadal Total Percentage Percentage Rate (%) Ratio (per Sq. Km) Growth Growth 1901 3289680 -- 919 42 97.66 -- 2.34 -- 1911 3848617 16.99 915 49 97.59 16.91 2.41 20.55 1921 4636980 20.48 896 59 97.26 20.08 2.74 36.80 1931 5560371 19.91 874 71 97.08 19.70 2.92 27.58 1941 6694790 20.4 875 85 96.89 20.16 3.11 28.30 1951 8028856 19.93 868 102 95.71 18.46 4.29 65.73 1961 10837329 34.98 869 138 92.79 30.87 7.21 126.57 1971 14625152 34.95 896 186 91.18 32.62 8.82 65.01 1991 22414322 53.26 923 286 88.9 49.42 11.10 92.97 2001 26655528 18.92 935 340 87.10 16.51 12.90 38.24 2011 31205576 17.07 958 398 85.90 15.47 14.10 27.89 * 1981 Census was not held in Assam. -

Census of India 2001 General Population Tables Assam
CENSUS OF INDIA 2001 GENERAL POPULATION TABLES ASSAM (Tables A-1 to A-4) ~, !,,\~(1.,... ~ +, .._' 1/, Of \ ~ PEOPLE ORIENTED OFFICE OF THE DIRECTOR OF CENSUS OPERATIONS, ASSAM Data Product Number 18-012-2001 - Cen.Book "(E) (ii) CONTENTS I f~age I Preface v Figures at a Glance vii Map Relating to Administrative Divisions ix SECTION 1- GENERAL NOTE General Note' 3 Census Concepts and Definitions 12 SECTION 2 - TABLES Talile A-I : Number of Villages, Towns, Households, Population and Area Fly Leaf 19 Diagram regarding Area and percentage to Total Ar~a 25 Map relating to Rural and Urban Population by Sex, 2001 2Qt Map relating to Sex ratio' - 2001 27 Diagram regarding Area, India and States 2001 28 Diagram regarding Population, India and States - 2001 29 Diagram regarding Population, State and Districts - 2001 30 \ . Map relating to Density of Population 31 Statements 33-42 Table A-I' 43-55 Appendix 56 Table A-2 : Decadal Variation in Population Since 1901 ~~ ~ Statements 71-75 Table A-2 76-80 Diagram relating to Growth of Population 1901-2001 India and State 81 Appendix 83 Table A-3 : Villages by Population size class ~~ ~ Statements 86-91 Table A-3 92-11 I Appendix 112-1I 4 (iii) Page Table A-4 Towns and Urban Agglomerations Classified by Population size Class in 2001 with variation.since 'i901 ~ Fly Leaf 117 Dia'PI"am regarding Growth of Urban Population showing percentage (1901-2001) 119 Map showing Population of Towns in six size classes 2001 110 Map ,showing Urban Population 121 Alph:j.betical list of towns, 2001 123 Alph4betical list of Urban Agglomerations - 2001 127 I Statetilents 128-185 I Table 1\-4 186-203 Appen~-l 204-205 Appendif-2 206 (iv) , PREFACE r"f'le final population data and its basic charactel;istics presented in this publication are based on the .1 data captured through the Household Schedules during the Actual Census Enumeration of 2001 Census and also from other SO\lrces. -

SERVICE AREA VILLAGE LIST for SONITPUR and BISWANATH DISTRICT
SERVICE AREA VILLAGE LIST for SONITPUR AND BISWANATH DISTRICT COMPILLED BY LEAD BANK OFFICE SONITPUR AND BISWANATH DISTRICT UCO BANK TEZPUR,ASSAM PIN 784001 SERVICE AREA VILLAGES UNDER PUB CHAIDUAR DEV.BLOCK TOTAL NO. OF BANKS :8 TOTAL NO. OF VILLAGES :357 Banks Sl Villages Gaon Panchayat Remarks No. 1.A.G.V. Bank 1 Aliguri Pub Kalabari Hawajan Branch 2 Aliguri Pichola Subansiri (Part) 3 Amguri Pichola Subansiri(Part) 4 Bejiachuk Pub Kalabari 5 Borachuk Dakhin Kalabari(Part) 6 Borachuk Kaibarta Dakhin Kalabari(Part) 7 Brahmangaon Pub Kalabari 8 Chapori Pichola Subansiri(Part) 9 Chengmaraguri Kalabari(Part) 10 Dakchaporib Kaibarta Pichola Subansiri(Part) 11 Deurichukgaon Pub Kalabari 12 Deurigaon Pub Kalabari 13 Dharakara Pichola Subansiri(Part) 14 Dologuri Dakhin Kalabari(Part) 15 Gamariati Pichola Subansiri(Part) 16 Ghuriagaon Pub Kalabari 17 Gitang Pichola Subansiri(Part) 18 Jokhinichuk Dakhin Kalabari(Part) 19 Kalitagaon Kalabari(Part) 20 Kardongchuk Pichola Subansiri(Part) 21 KHaraiguri Pub Kalabari 22 Khutikatia gaon Pub Kalabari 23 Kukurashowa Kalabari(Part) 24 Maj Pichala Pichola Subansiri(Part) 25 Mohkhali Pichola Subansiri(Part) 26 No.1 Dola Dakhin Kalabari(Part) 27 No.1 Satiara Pub Kalabari 28 No.2 Deurigaon Pub Kalabari 29 No.2 Dalla Doloni Kalabari(Part) 30 No.1 Kolmouguri Sessa Asomiya Kalabari(Part) 31 Nopamua Pub Kalabari 32 Pichala Dakchapori Pichola Subansiri(Part) 33 Pichalamukh Pub Kalabari 34 Salamora Dakhin Kalabari(Part) 35 Satiara Pichola Subansiri(Part) 36 Satiara Pathar Pub Kalabari 37 Sowaguri Pichola Subansiri(Part) 38 Sutargaon Pub Kalabari 39 Tamulibheti Pub Kalabari Banks Sl Villages Gaon Panchayat Remarks No. 2. A.G.V. -

CARRICULUM VITAE of SWAPNA KAKATI,Assistant Professor in History,Chaiduar College,Gohpur,Assam
CARRICULUM VITAE of SWAPNA KAKATI,Assistant Professor in History,Chaiduar College,Gohpur,Assam. 1. Name & Designation :Swapna Kakati, Assistant Professor. 2. Husband’s Name : Dr. Mohini Mohan Borah 3. Date of Birth :01-03-1975. 4. Sex :Female. 5. Nationality :Indian. 6. Religion :Hinduism. 7. Caste :General. 8. Educational Qualification : M.A., B. Ed., M. Phil. 9. Other Qualification : CCA 10. Subject :History. 11. Special Group :Ancient Group. 12 . Present Address : Wardno.-6, Madhya Chatrang, P.O.-Gohpur, Dist.-Sonitpur,Assam, PIN-784168. 13. Permanent Address : -Do- 14 . Examination passed: Name of Year of Institutions Board / Results % of Exams Passing University Marks. H.S.L.C. 1990 Hawajan H.S. SEBA I – 80.6% School. Div. H.S.S.L.C. 1992 Cotton College AHSEC II – 59 % (Science) Div B.A. 1995 Handique Girls’ G.U. II – 57 % (Major) College Class M.A. 1997 Gauhati University G.U. I – 61 % (History) Class B.Ed 1998 Chaiduar Shikshan G.U. II – 57 % Mahavidyalay Class C.C.A. 1998 C.S.I., GHY G.U. I – 64 % Class M .Phil 2008 V. M. University, V.M.University, I-Class 63% Salem, Tamilnadu Salem, Tamilnadu 15. Academic Experience : (a) Teaching Experience : 17 Years (b) Research Experience : 12 Years. (c) No. of Publications : 68 (i)International : 06 (ii)National : 32 (iii)Popular Articles : 20 (iv) Text Books : 08 (v) Reference Books : 01 (vi) Edited Books) : 01 (d) No. of Seminar/Workshop: 35 /OC/RC Attended. 16. No. of Research Project : 03 Undertaken & Funding Agengies. Name of Status Amount Funding Title Project Received Agency UGC, NE Temple Architecture, Regional Sculpture And Painting 1. -

ASSAM ACCESSIBLE the Gateway to the North East, Assam Has Seen Rapid Progress in Road Infrastructure Development Since 2014
MAKING ASSAM ACCESSIBLE The Gateway to the North East, Assam has seen rapid progress in road infrastructure development since 2014. The length of National Highways in the State has reached 3,909 km in 2018. India’s longest bridge, the 9.15 km long Bhupen Hazarika Setu worth Rs. 876 Cr across River Brahmaputra, connecting the Dhola and Sadiya ghats of Assam and Arunachal Pradesh was dedicated to the nation by Prime Minister Narendra Modi in May 2017. Over 300 km of National Highways are being upgraded to 4 lanes, at a cost of over Rs. 10,000 Cr. With engineering marvel projects like Dhubri-Phulbari Bridge & Gohpur-Tumligarh over Brahmaputra being built, the State’s economy is destined to fast track in coming years. “When a network of good roads is created, the economy of the country also picks up pace. Roads are veins and arteries of the nation, which help to transform the pace of development and ensure that prosperity reaches the farthest corners of our nation.” NARENDRA MODI Prime Minister “In the past four years, we have expanded the length of Indian National Highways network to 1,26,350 km. The highway sector in the country has seen a 20% growth between 2014 and 2018. Tourist destinations have come closer. Border, tribal and backward areas are being connected seamlessly. Multimodal integration through road, rail and port connectivity is creating socio economic growth and new opportunities for the people. In the coming years, we have planned projects with investments worth over Rs 6 lakh crore, to further expand the world’s second largest road network.” NITIN GADKARI Union Minister, Ministry of Road Transport & Highways, Shipping and Water Resources, River Development & Ganga Rejuvenation Fast tracking National Highway development in Assam NH + IN PRINCIPLE NH LENGTH UPTO YEAR 2018 3,909 km NH LENGTH UPTO YEAR 2014 3,634 km Adding new National Highways in Assam 1,945 km 798 km Yr 2014 - 2018 Yr 2010 - 2014 New NH New NH & In principle NH length 6 Road Projects awarded in Assam Yr 2010 - 2014 Yr 2014 - 2018 681 km 1,139.77 km Total Cost Total Cost Rs. -
Internal Displacement, Migration, and Policy in Northeastern India
No. 8, April 2007 InternalȱDisplacement,ȱ Migration,ȱandȱPolicyȱinȱ PAPERS ȱ NortheasternȱIndia Uddipana Goswami East-West Center Washington WORKING EastȬWestȱCenterȱ TheȱEastȬWestȱCenterȱisȱanȱ internationallyȱrecognizedȱeducationȱ andȱresearchȱorganizationȱ establishedȱbyȱtheȱU.S.ȱCongressȱinȱ 1960ȱtoȱstrengthenȱunderstandingȱ andȱrelationsȱbetweenȱtheȱUnitedȱ StatesȱandȱtheȱcountriesȱofȱtheȱAsiaȱ Pacific.ȱThroughȱitsȱprogramsȱofȱ cooperativeȱstudy,ȱtraining,ȱ seminars,ȱandȱresearch,ȱtheȱCenterȱ worksȱtoȱpromoteȱaȱstable,ȱpeacefulȱ andȱprosperousȱAsiaȱPacificȱ communityȱinȱwhichȱtheȱUnitedȱ Statesȱisȱaȱleadingȱandȱvaluedȱ partner.ȱFundingȱforȱtheȱCenterȱ comesȱforȱtheȱU.S.ȱgovernment,ȱ privateȱfoundations,ȱindividuals,ȱ corporationsȱandȱaȱnumberȱofȱAsiaȬ Pacificȱgovernments.ȱȱ EastȬWestȱCenterȱWashington EstablishedȱonȱSeptemberȱ1,ȱ2001,ȱtheȱ primaryȱfunctionȱofȱtheȱEastȬWestȱ CenterȱWashingtonȱisȱtoȱfurtherȱtheȱ EastȬWestȱCenterȱmissionȱandȱtheȱ institutionalȱobjectiveȱofȱbuildingȱaȱ peacefulȱandȱprosperousȱAsiaȱPacificȱ communityȱthroughȱsubstantiveȱ programmingȱactivitiesȱfocusedȱonȱ theȱthemeȱofȱconflictȱreductionȱinȱtheȱ AsiaȱPacificȱregionȱandȱpromotingȱ Americanȱunderstandingȱofȱandȱ engagementȱinȱAsiaȱPacificȱaffairs. ContactȱInformation: Editor,ȱEWCWȱWorkingȱPapers EastȬWestȱCenterȱWashington 1819ȱLȱStreet,ȱNW,ȱSuiteȱ200 Washington,ȱD.C.ȱȱ20036 Tel:ȱ(202)ȱ293Ȭ3995 Fax:ȱ(202)ȱ293Ȭ1402 [email protected] Uddipana Goswamiȱis a Ph.D. fellow at the Centre for Studies in Social Sciences, Calcutta (CSSSCAL). EastȬWestȱCenterȱWashingtonȱWorkingȱPapers -
Pre-Feasibility Report
Pre-Feasibility Report For Proposed Construction of four lane TBM TUNNEL including approaches under River Brahmaputra between Gohpur on NH-52 North Bank and Numaligarh on NH-37 South Bank, Assam (Length: 34.664 km) on EPC Mode under SARDP-NE Phase-A Project Proponent “National Highways & Infrastructure Development Corporation Ltd. (NHIDCL)” Prepared By National Highways & Infrastructure Development Corporation Ltd. Pre-Feasibility Report - Proposed Construction of four lane TBM TUNNEL including approaches under River Brahmaputra between Gohpur on NH-52 North Bank and Numaligarh on NH-37 South Bank, Assam (Length: 34.664 km) on EPC Mode under SARDP-NE Phase-A TABLE OF CONTENTS 1. EXECUTIVE SUMMARY ....................................................................................................... 1 2. INTRODUCTION OF PROJECT/BACKGROUND INFORMATION ............................. 2 2.1 Identification of Project & Project Proponent .................................................................. 2 2.2 Description of Nature of Project ....................................................................................... 2 2.3 Need for the project .......................................................................................................... 3 2.4 Demand & Supply Gap ..................................................................................................... 4 2.5 Import vs Indigenous Production ..................................................................................... 4 2.6 Export Possibilities ..........................................................................................................