Statistics on Narcotic Drugs for 1968
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Minnesota Statutes 1979 Supplement
MINNESOTA STATUTES 1979 SUPPLEMENT 152.01 PROHIBITED DRUGS CHAPTER 152. PROHIBITED DRUGS Sec. 152.01 Definitions. 152.02 Schedules of controlled substances; admin istration of chapter. 152.01 Definitions. [For text of subds 1 to 8, see M.S.1978] Subd. 9. Marijuana. "Marijuana" means all parts of the plant of any species of the genus Cannabis, including all agronomical varieties, whether growing or not; the seeds thereof; the resin extracted from any part of such plant; and every compound, manufacture, salt, derivative, mixture, or preparation of such plant, its seeds or resin, but shall not include the mature stalks of such plant, fiber from such stalks, oil or cake made from the seeds of such plant, any other compound, manufacture, salt, derivative, mix ture, or preparation of such mature stalks, except the resin extracted therefrom, fiber, oil, or cake, or the sterilized seed of such plant which is incapable of germination. [For text of subds 10 to 17, see M.S.1978] [ 1979 c 157 s 1 ] 152.02 Schedules of controlled substances; administration of chapter. [For text of subd 1, see M.S.1978) Subd. 2. The following items are listed in Schedule I: (1) Any of the following substances, including their isomers, esters, ethers, salts, and salts of isomers, esters, and ethers, unless specifically excepted, whenever the exis tence of such isomers, esters, ethers and salts is possible within the specific chemical des ignation: Acetylmethadol; Allylprodine; Alphacetylmethadol; Alphameprodine; Alpham- ethadol; Benzethidine; Betacetylmethadol; Betameprodine; Betamethadol; Betaprodine; Clonitazene; Dextromoramide; Dextrorphan; Diampromide; Diethyliambutene; Dime- noxadol; Dimepheptanol; Dimethyliambutene; Dioxaphetyl butyrate; Dipipanone; Ethylmethylthiambutene; Etonitazene; Etoxeridine; Furethidine; Hydroxypethidine; Ke- tobemidone; Levomoramide; Levophenacylmorphan; Morpheridine; Noracymethadol; Norlevorphanol; Normethadone; Norpipanone; Phenadoxone; Phenampromide; Pheno- morphan; Phenoperidine; Piritramide; Proheptazine; Properidine; Racemoramide; Tri meperidine. -

2020 Kansas Statutes
2020 Kansas Statutes 65-4105. Substances included in schedule I. (a) The controlled substances listed in this section are included in schedule I and the number set forth opposite each drug or substance is the DEA controlled substances code that has been assigned to it. (b) Any of the following opiates, including their isomers, esters, ethers, salts, and salts of isomers, esters and ethers, unless specifically excepted, whenever the existence of these isomers, esters, ethers and salts is possible within the specific chemical designation: (1) Acetyl fentanyl (N-(1-phenethylpiperidin-4-yl)-N- phenylacetamide) 9821 (2) Acetyl-alpha-methylfentanyl (N-[1-(1-methyl-2-phenethyl)-4-piperidinyl]-N- phenylacetamide) 9815 (3) Acetylmethadol 9601 (4) Acryl fentanyl (N-(1-phenethylpiperidin-4-yl)-N-phenylacrylamide; acryloylfentanyl) 9811 (5) AH-7921 (3,4-dichloro-N-[(1-dimethylamino)cyclohexylmethyl]benzamide) 9551 (6) Allylprodine 9602 (7) Alphacetylmethadol 9603(except levo-alphacetylmethadol also known as levo- alpha-acetylmethadol, levomethadyl acetate or LAAM) (8) Alphameprodine 9604 (9) Alphamethadol 9605 (10) Alpha-methylfentanyl (N-[1-(alpha-methyl-beta-phenyl)ethyl-4-piperidyl] propionanilide; 1-(1-methyl-2-phenylethyl)-4-(N-propanilido) piperidine) 9814 (11) Alpha-methylthiofentanyl (N-[1-methyl-2-(2-thienyl)ethyl-4-piperidinyl]-N- phenylpropanamide) 9832 (12) Benzethidine 9606 (13) Betacetylmethadol 9607 (14) Beta-hydroxyfentanyl (N-[1-(2-hydroxy-2-phenethyl)-4-piperidinyl]-N- phenylpropanamide) 9830 (15) Beta-hydroxy-3-methylfentanyl (other -

2015-02 Toxicology Rapid Testing Panel
SOUTH CAROLINA LAW ENFORCEMENT DIVISION NIKKI R. HALEY MARK A. KEEL Governor Chief FORENSIC SERVICES LABORATORY CUSTOMER NOTICE 2015-02 REGARDING TOXICOLOGY RAPID TESTING PANEL August 12, 2015 This notice is to inform the Coroners of South Carolina of a new testing panel available through the SLED Toxicology Department. On Monday, August 17th, the Toxicology Department will begin offering both a Rapid Testing Panel in addition to the already available Expanded Testing Panel. This Rapid Testing Panel is to be utilized in cases where the Expanded Testing Panel is not warranted, specifically where a cause of death has already been established. The Rapid Testing Panel will consist of volatiles analysis, to include, ethanol, acetone, isopropanol and methanol, drug screens, and drug confirmation/quantitation of positive screens. The cases assigned to the Rapid Testing Panel will have an expedited turnaround time. Targeted turn around times will be two weeks for negative cases and six weeks or less for positive cases. While every effort will be made to adhere to these time frames, additional time may be required on occasion due to the nature of postmortem samples. Submitters will be notified if there is a problem with a particular sample. Please see attachment regarding specifically which substances are covered by the Rapid Testing Panel and the Expanded Testing Panel. As always, a detailed case history and list of drugs suspected is appreciated. Rapid Panel and Expanded Panel will be choices available in iLAB. Please contact Lt. Dustin Smith (803-896-7385) with additional questions. ALI-359-T An Accredited Law Enforcement Agency P.O. -

Properties and Units in Clinical Pharmacology and Toxicology
Pure Appl. Chem., Vol. 72, No. 3, pp. 479–552, 2000. © 2000 IUPAC INTERNATIONAL FEDERATION OF CLINICAL CHEMISTRY AND LABORATORY MEDICINE SCIENTIFIC DIVISION COMMITTEE ON NOMENCLATURE, PROPERTIES, AND UNITS (C-NPU)# and INTERNATIONAL UNION OF PURE AND APPLIED CHEMISTRY CHEMISTRY AND HUMAN HEALTH DIVISION CLINICAL CHEMISTRY SECTION COMMISSION ON NOMENCLATURE, PROPERTIES, AND UNITS (C-NPU)§ PROPERTIES AND UNITS IN THE CLINICAL LABORATORY SCIENCES PART XII. PROPERTIES AND UNITS IN CLINICAL PHARMACOLOGY AND TOXICOLOGY (Technical Report) (IFCC–IUPAC 1999) Prepared for publication by HENRIK OLESEN1, DAVID COWAN2, RAFAEL DE LA TORRE3 , IVAN BRUUNSHUUS1, MORTEN ROHDE1, and DESMOND KENNY4 1Office of Laboratory Informatics, Copenhagen University Hospital (Rigshospitalet), Copenhagen, Denmark; 2Drug Control Centre, London University, King’s College, London, UK; 3IMIM, Dr. Aiguader 80, Barcelona, Spain; 4Dept. of Clinical Biochemistry, Our Lady’s Hospital for Sick Children, Crumlin, Dublin 12, Ireland #§The combined Memberships of the Committee and the Commission (C-NPU) during the preparation of this report (1994–1996) were as follows: Chairman: H. Olesen (Denmark, 1989–1995); D. Kenny (Ireland, 1996); Members: X. Fuentes-Arderiu (Spain, 1991–1997); J. G. Hill (Canada, 1987–1997); D. Kenny (Ireland, 1994–1997); H. Olesen (Denmark, 1985–1995); P. L. Storring (UK, 1989–1995); P. Soares de Araujo (Brazil, 1994–1997); R. Dybkær (Denmark, 1996–1997); C. McDonald (USA, 1996–1997). Please forward comments to: H. Olesen, Office of Laboratory Informatics 76-6-1, Copenhagen University Hospital (Rigshospitalet), 9 Blegdamsvej, DK-2100 Copenhagen, Denmark. E-mail: [email protected] Republication or reproduction of this report or its storage and/or dissemination by electronic means is permitted without the need for formal IUPAC permission on condition that an acknowledgment, with full reference to the source, along with use of the copyright symbol ©, the name IUPAC, and the year of publication, are prominently visible. -

Pjp3'2001.Vp:Corelventura
Copyright © 2001 by Institute of Pharmacology Polish Journal of Pharmacology Polish Academy of Sciences Pol. J. Pharmacol., 2001, 53, 253261 ISSN 1230-6002 INFLUENCE OF DOXEPIN USED IN PREEMPTIVE ANALGESIA ON THE NOCICEPTION IN THE PERIOPERATIVE PERIOD. EXPERIMENTAL AND CLINICAL STUDY Jerzy Wordliczek, Marcin Banach, Magdalena Dorazil, Barbara Przew³ocka*,# Department of Anaesthesiology and Intensive Care, 1st Chair of General Surgery of Collegium Medicum, Jagiellonian University, Kopernika 17, PL 31-501 Kraków, Poland, *Department of Molecular Neuropharmacology, Institute of Pharmacology, Polish Academy of Sciences, Smêtna 12, PL 31-343 Kraków, Poland Influence of doxepin used in preemptive analgesia on the nociception in the perioperative period. Experimental and clinical study. J. WORDLI- CZEK, M. BANACH, M. DORAZIL, B. PRZEW£OCKA. Pol. J. Pharma- col., 2001, 53, 253–261. The aim of the present research was to assess in experimental and clini- cal study the influence of doxepin administered intraperitoneally (ip) as pre- emptive analgesia on the nociception in the perioperative period. The pain thresholds for mechanical stimuli were measured in rats. The objective of clinical investigation was to assess the influence of preemptive administra- tion of doxepin on postoperative pain intensity, analgesic requirement in the early postoperative period as well as an assessment of the quality of postope- rative analgesia by the patient. Doxepin injected ip (3–30 mg/kg) dose-dependently increased the pain threshold for mechanical stimuli measured in paw pressure test in rats. Do- xepin injected 30 min before formalin significantly increased the nociceptive threshold in the paw pressure test. In contrast, doxepin injected 240 min before formalin or 10 min after formalin did not change the nociceptive threshold. -

Pharmacy and Poisons (Third and Fourth Schedule Amendment) Order 2017
Q UO N T FA R U T A F E BERMUDA PHARMACY AND POISONS (THIRD AND FOURTH SCHEDULE AMENDMENT) ORDER 2017 BR 111 / 2017 The Minister responsible for health, in exercise of the power conferred by section 48A(1) of the Pharmacy and Poisons Act 1979, makes the following Order: Citation 1 This Order may be cited as the Pharmacy and Poisons (Third and Fourth Schedule Amendment) Order 2017. Repeals and replaces the Third and Fourth Schedule of the Pharmacy and Poisons Act 1979 2 The Third and Fourth Schedules to the Pharmacy and Poisons Act 1979 are repealed and replaced with— “THIRD SCHEDULE (Sections 25(6); 27(1))) DRUGS OBTAINABLE ONLY ON PRESCRIPTION EXCEPT WHERE SPECIFIED IN THE FOURTH SCHEDULE (PART I AND PART II) Note: The following annotations used in this Schedule have the following meanings: md (maximum dose) i.e. the maximum quantity of the substance contained in the amount of a medicinal product which is recommended to be taken or administered at any one time. 1 PHARMACY AND POISONS (THIRD AND FOURTH SCHEDULE AMENDMENT) ORDER 2017 mdd (maximum daily dose) i.e. the maximum quantity of the substance that is contained in the amount of a medicinal product which is recommended to be taken or administered in any period of 24 hours. mg milligram ms (maximum strength) i.e. either or, if so specified, both of the following: (a) the maximum quantity of the substance by weight or volume that is contained in the dosage unit of a medicinal product; or (b) the maximum percentage of the substance contained in a medicinal product calculated in terms of w/w, w/v, v/w, or v/v, as appropriate. -

S1 Table. List of Medications Analyzed in Present Study Drug
S1 Table. List of medications analyzed in present study Drug class Drugs Propofol, ketamine, etomidate, Barbiturate (1) (thiopental) Benzodiazepines (28) (midazolam, lorazepam, clonazepam, diazepam, chlordiazepoxide, oxazepam, potassium Sedatives clorazepate, bromazepam, clobazam, alprazolam, pinazepam, (32 drugs) nordazepam, fludiazepam, ethyl loflazepate, etizolam, clotiazepam, tofisopam, flurazepam, flunitrazepam, estazolam, triazolam, lormetazepam, temazepam, brotizolam, quazepam, loprazolam, zopiclone, zolpidem) Fentanyl, alfentanil, sufentanil, remifentanil, morphine, Opioid analgesics hydromorphone, nicomorphine, oxycodone, tramadol, (10 drugs) pethidine Acetaminophen, Non-steroidal anti-inflammatory drugs (36) (celecoxib, polmacoxib, etoricoxib, nimesulide, aceclofenac, acemetacin, amfenac, cinnoxicam, dexibuprofen, diclofenac, emorfazone, Non-opioid analgesics etodolac, fenoprofen, flufenamic acid, flurbiprofen, ibuprofen, (44 drugs) ketoprofen, ketorolac, lornoxicam, loxoprofen, mefenamiate, meloxicam, nabumetone, naproxen, oxaprozin, piroxicam, pranoprofen, proglumetacin, sulindac, talniflumate, tenoxicam, tiaprofenic acid, zaltoprofen, morniflumate, pelubiprofen, indomethacin), Anticonvulsants (7) (gabapentin, pregabalin, lamotrigine, levetiracetam, carbamazepine, valproic acid, lacosamide) Vecuronium, rocuronium bromide, cisatracurium, atracurium, Neuromuscular hexafluronium, pipecuronium bromide, doxacurium chloride, blocking agents fazadinium bromide, mivacurium chloride, (12 drugs) pancuronium, gallamine, succinylcholine -

Drugs of Abuseon September Archived 13-10048 No
U.S. DEPARTMENT OF JUSTICE DRUG ENFORCEMENT ADMINISTRATION WWW.DEA.GOV 9, 2014 on September archived 13-10048 No. v. Stewart, in U.S. cited Drugs of2011 Abuse EDITION A DEA RESOURCE GUIDE V. Narcotics WHAT ARE NARCOTICS? Also known as “opioids,” the term "narcotic" comes from the Greek word for “stupor” and originally referred to a variety of substances that dulled the senses and relieved pain. Though some people still refer to all drugs as “narcot- ics,” today “narcotic” refers to opium, opium derivatives, and their semi-synthetic substitutes. A more current term for these drugs, with less uncertainty regarding its meaning, is “opioid.” Examples include the illicit drug heroin and pharmaceutical drugs like OxyContin®, Vicodin®, codeine, morphine, methadone and fentanyl. WHAT IS THEIR ORIGIN? The poppy papaver somniferum is the source for all natural opioids, whereas synthetic opioids are made entirely in a lab and include meperidine, fentanyl, and methadone. Semi-synthetic opioids are synthesized from naturally occurring opium products, such as morphine and codeine, and include heroin, oxycodone, hydrocodone, and hydromorphone. Teens can obtain narcotics from friends, family members, medicine cabinets, pharmacies, nursing 2014 homes, hospitals, hospices, doctors, and the Internet. 9, on September archived 13-10048 No. v. Stewart, in U.S. cited What are common street names? Street names for various narcotics/opioids include: ➔ Hillbilly Heroin, Lean or Purple Drank, OC, Ox, Oxy, Oxycotton, Sippin Syrup What are their forms? Narcotics/opioids come in various forms including: ➔ T ablets, capsules, skin patches, powder, chunks in varying colors (from white to shades of brown and black), liquid form for oral use and injection, syrups, suppositories, lollipops How are they abused? ➔ Narcotics/opioids can be swallowed, smoked, sniffed, or injected. -

Guidelines for Ensuring Patient Access To, and Safe Management Of, Controlled Medicines Notice
African Palliative Care Association Guidelines for Ensuring Patient Access to, and Safe Management of, Controlled Medicines Notice Medicine is an ever-changing science. As new research and clinical experience broaden our knowledge, changes in treatment and drug therapy are required. APCA and the publisher of this work have checked with sources believed to be reliable in their efforts to provide information that is complete and generally in accord with the standards accepted at the time of this publication. However, in view of the possibility of human error or changes in sciences, neither APCA nor the publisher nor any other party who have been involved in the preparation or publication of this work warrants that the information contained in this publication is complete and correct and they disclaim all responsibility for any errors or omission or for the results obtained from use of the information contained herein. African Palliative Care Association Foreword The 59th Session of the World Health conventions. Several countries make the Assembly of the UN adopted resolution importation, storage, distribution and 58.22, thereby recognising the dispensing of controlled medicines more importance of improving pain relief restrictive than is needed. Additionally, using opioid analgesics and calling on it is a requirement for countries to member states to remove barriers to provide detailed annual estimates and their medical use and availability. reports for narcotic substances to the International Narcotic Control Board to While advances have been made pursuing procure or produce controlled medicines. this agenda in Africa (e.g. legalisation of However, formulating reliable estimates oral morphine prescription rights for nurses is often a barrier to accessing controlled and clinical officers in Uganda, approval medicines, while the procurement of access to morphine for hospices of opioids is subject to complex and in Zambia, and advocacy progress in lengthy exportation and importation Malawi and Kenya), challenges remain. -

Narcotic Drugs and Psychotropic Substances (Control) Act
LAWS OF GUYANA Narcotic Drugs and Psychotropic Cap. 35:11 3 Substances (Control) CHAPTER 35:11 NARCOTIC DRUGS AND PSYCHOTROPIC SUBSTANCES (CONTROL) ACT ARRANGEMENT OF SECTIONS PART I PRELIMINARY SECTION 1. Short title. 2. Interpretation. PART II PROHIBITION OF POSSESSION OF AND TRAFFICKING IN NARCOTICS AND CULTIVATION OF CERTAIN PLANTS 3. Definitions for Part II. 4. Penalty for possession of narcotic. 5. Penalty for trafficking in narcotic. 6. Penalty for supply, etc., of narcotic to child or young person if death results from consumption or administration of it. 7. Penalty for bringing into prison or taking out of prison, etc., of a narcotic. 8. Penalty for cultivation of certain plants. 9. Procedure for purposes of section 8(2) to (6). 10. Power of entry in respect of State or Government lands. 11. Power of destruction of prohibited plants. 12. Penalty for certain other acts connected with narcotics. 13. Certain prescriptions to be unlawful. 14. Penalty for receiving additional narcotic or prescription without disclosure of earlier receipt. 15. Removal of name from register. L.R.O. 3/1998 LAWS OF GUYANA 4 Cap. 35:11 Narcotic Drugs and Psychotropic Substances (Control) PART III NARCOTICS IN TRANSIT SECTION 16. Definitions for Part III. 17. Prohibition against sending narcotics by post. 18. Narcotics in transit. 19. Control of Comptroller over narcotics brought into Guyana in transit. 20. Tampering with narcotics in transit. 21. Diversion in Guyana of narcotics in transit. 22. Variations in export authorisation, import authorisation or diversion certificate granted in country other than Guyana. PART IV LICENCES 23. Grant and renewal of licences. -
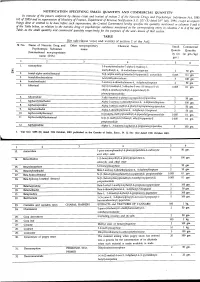
NOTIFICATION SPECIFYING SMALL QUANTITY and COMMERCIAL QUANTITYI Ly of the Pozoersconferred (Xxiiiil
NOTIFICATION SPECIFYING SMALL QUANTITY AND COMMERCIAL QUANTITYI ly of the pozoersconferred (xxiiiil . - lleyise by clansesfuiia) and of section2 of the Nnrcotic Drrtgsnnd pnlchotropic SrtbstancesAct, 19g5 (61 of 1985)and in supersessionof Ministry of Ftnnnce, (D Departmentof'Reuenue Noiification s.o.527 aotra i'6tt,1uli, tssi, exceptas respects things doneor omitted to be done .beforesuch supersession,the Cential Gooernmenihereby spectfiesthe qtnntitrl ,nlnt'ionedin coltnnnsS and 6 of the Tablebelow, in relationto.the narcoticdrug or psychotropicsubstnnce mentioned.ii tlr', ,1urrponding entnl in columns2 to 4 of thesaid Table,as the small quantitrl nnd commercialquantity respectiailyfor the purposesof the said clnuse:sof ttrit seciion. TABLE [Seesub-clause vii(a) and xxiii(a) of section 2 of the Act] Sl No. Name of Narcotic Drug and Other non-proprietary Chemical Name Small Commercial Psychotropic Substance name Quanti- Quantity (Intemational non-proprietary ity (in (in gm./kg.) name (INN) Acetorphine 3-0-acetyltetrahydro-7-alpha-(l-hydroxy-l- methylbutyt)-o, l4-endoetheno-onpavine $ 50 9.. 1\) Acetyl-alpha-methylfen N-[-(alpha-methylphenethyl)-4-piperidy]l acetanilide 0.1 g-. J. Acetyldihydrocodeine 100 4. Acetylmethadol 3-acetoxy-6-dimethylamino-4, 4 lheptane 50 gm. 5. Alfentanil -ethyl-4, N-[1-[2-( 5-dihydro-S-oxo-lH-tetrazol-t-yt) 01 gm. ethyll-4-(methoxymethyl)-4-piperidinyll -N- ylpropanamide Allyprodine 3-allyl Jmethyl-4-phenyl-4 Alpha-3-acetoxy-6-dimethylamino-4,Ld lheptane 100 gm Alpha-3-ethyl-l-methyl-4-phenyl-4-propionox 50 gm. 9.7' AlphamethadolArPnametnaool Alpha-6-dimethylamino-4, 4-diphenyl-3-heptanol 2 S0 gm. 10. Alphu-*"thylf"ntur,yl 11. -

The International Drug Control Conventions
ST/CND/1/Add.1/Rev.3 The International Drug Control Conventions Schedules of the Single Convention on Narcotic Drugs of 1961 as amended by the 1972 Protocol, as at 22 April 2017 UNITED NATIONS New York, 2017 ST/CND/1/Add.1/Rev.3 © United Nations, 2017. All rights reserved, worldwide. Schedules of the Single Convention on Narcotic Drugs of 1961 as amended by the 1972 Protocol, as at 22 April 2017 List of drugs included in Schedule I Acetorphine 3-O-Acetyltetrahydro-7α-(1-hydroxy-1-methylbutyl)- 6,14-endo-ethenooripavine Acetyl-alpha-methylfentanyl N-[1-(α-Methylphenethyl)-4-piperidyl]acetanilide Acetylfentanyl N-phenyl-N-[1-(2-phenylethyl)-4-piperidinyl]acetamide Acetylmethadol 3-Acetoxy-6-dimethylamino-4,4-diphenylheptane AH-7921 3,4-dichloro-N-{[1- (dimethylamino)cyclohexyl]methyl}benzamide Alfentanil N-[1-[2-(4-Ethyl-4,5-dihydro-5-oxo-1H-tetrazol-1-yl) ethyl]-4-(methoxymethyl)-4-piperidinyl]-N- phenylpropanamide Allylprodine 3-Allyl-1-methyl-4-phenyl-4-propionoxypiperidine Alphacetylmethadol α-3-Acetoxy-6-dimethylamino-4,4-diphenylheptane Alphameprodine α-3-Ethyl-1-methyl-4-phenyl-4-propionoxypiperidine Alphamethadol α-6-Dimethylamino-4,4-diphenyl-3-heptanol alpha-Methylfentanyl N-[1-(α-Methylphenethyl)-4-piperidyl]propionanilide alpha-Methylthiofentanyl N-[1-[1-Methyl-2-(2-thienyl)ethyl]-4-piperidyl] propionanilide Alphaprodine α-l,3-Dimethyl-4-phenyl-4-propionoxypiperidine Anileridine 1-p-Aminophenethyl-4-phenylpiperidine-4-carboxylic acid ethyl ester Benzethidine 1-(2-Benzyloxyethyl)-4-phenylpiperidine-4-carboxylic acid ethyl ester