The Specter of Genetically Engineered Animals
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

VIA CERTIFIED MAIL RETURN RECEIPT REQUESTED September 26, 2013 Secretary Vilsack U.S. Department of Agriculture (USDA) Animal He
VIA CERTIFIED MAIL RETURN RECEIPT REQUESTED September 26, 2013 Secretary Vilsack U.S. Department of Agriculture (USDA) Animal Health and Plant Inspection Service (APHIS) 1400 Independence Ave., S.W. Washington, DC 20250 Petition for Agency Action, Rulemaking, Investigation, and Otherwise Collateral Relief on Recent Genetically Engineered Alfalfa Contamination Introduction Pursuant to the Right to Petition the Government Clause of the United States Constitution,1 the Administrative Procedure Act (APA),2 and United States Department of Agriculture (USDA)’s implementing regulations,3 petitioner Center for Food Safety (CFS) respectfully submits this petition for agency action, rulemaking, and collateral relief on behalf of its over 350,000 farmer and consumer members, including alfalfa farmers Joseph and Michelle Peila. CFS requests that the Department retract its September 17, 2013 decision and take all regulatory action needed to remedy the current genetically engineered (GE) alfalfa contamination because its original decision was based on erroneous factual information—that the transgenic contamination occurred after GE Roundup Ready alfalfa was approved for commercial sale. In fact, records indicate that the seed used for the rejected contaminated alfalfa itself tested positive for Roundup Ready contamination, and this seed was purchased in 2010, before the deregulation of Roundup Ready alfalfa. CFS is a nationwide public interest non-profit membership organization with offices in Washington, D.C., San Francisco, CA, and Portland, OR. Since the organization’s founding in 1997, CFS has sought to ameliorate the adverse impacts of industrial farming and food production systems on human health, animal welfare, and the environment. CFS also supports and promotes sustainable forms of agriculture, including organic systems. -

Position Statement on Genetically Engineered Food
Position Statement on Genetically Engineered Food ealth Care Without Harm opposes the produc- Human Health Concerns tion and marketing of genetically engineered Few long-term studies have been conducted to assure that H(GE) foods. These foods are not adequately production and consumption of GE foods will carry no assessed for their credible adverse effects on human or adverse long-term health impacts. A 2003 peer-reviewed animal health, or on the environment in which they are literature search found just ten published studies specifi- produced. Also of concern is the threat posed by genetic cally designed to assess the potential for health effects engineering to environmentally sustainable food produc- from GE foods or feed.4 For hospitals, patient health is tion and the threat to the economic livelihood of farmers of particular concern, since some patients may be more pursing sustainable food production. vulnerable to possible problems from GE foods than the We therefore encourage health care providers to purchase general public. For example, full digestion of proteins de- non-GE foods to the extent possible and to source from creases the likelihood they will survive to produce harm suppliers that demonstrate a strong commitment to al- (via direct toxicity or allergenicity), whereas digestive ternatives to GE food, and that support local farmers and function is sometimes compromised in hospital patients. sustainable practices. The immune system in such patients may also be compro- mised, making them more generally susceptible to harm. Background Allergies: Genetic engineering moves proteins novel For about a decade,1 companies have introduced geneti- to the human diet into the food supply. -

UNITED STATES SECURITIES and EXCHANGE COMMISSION Washington, D.C
UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 10-K ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the fiscal year ended December 31, 2018 OR TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the transition period from to . Commission File Number: 001-36042 INTREXON CORPORATION (Exact name of registrant as specified in its charter) Virginia 26-0084895 (State or other jurisdiction of (I.R.S. Employer incorporation or organization) Identification Number) 20374 Seneca Meadows Parkway Germantown, Maryland 20876 (Address of principal executive offices) (Zip Code) Registrant's telephone number, including area code (301) 556-9900 Securities registered pursuant to Section 12(b) of the Act: Title of each class Name of each exchange on which registered Intrexon Corporation Common Stock, No Par Value Nasdaq Global Select Market Securities registered pursuant to Section 12(g) of the Act: None Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes No Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes No Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. -
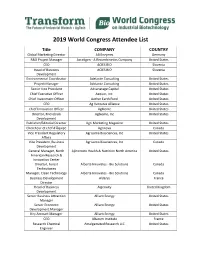
2019 World Congress Attendee List
2019 World Congress Attendee List Title COMPANY COUNTRY Global Marketing Director AB Enzymes Germany R&D Project Manager Acceligen - A Recombinetics Company United States CEO ACIES BIO Slovenia Head of Business ACIES BIO Slovenia Development Environmental Coordinator Adelante Consulting United States Project Manager Adelante Consulting United States Senior Vice President Advanatage Capital United States Chief Executive Officer Aequor, Inc. United States Chief Investment Officer Aether Earth Fund United States CEO Ag Ventures Alliance United States Chief Innovation Officer AgBiome United States Director, Microbials Agbiome, Inc United States Development Publisher/Editorial Director Agri Marketing Magazine United States Chercheur et chef d'Ãquipe Agrinova Canada Vice President Regulatory Agrisoma Biosciences, Inc United States Affairs Vice President, Business Agrisoma Biosciences, Inc Canada Development General Manager, North Ajinomoto Health & Nutrition North America United States American Research & Innovation Center Director, Forest Alberta Innovates - Bio Solutions Canada Technologies Manager, Clean Technology Alberta Innovates - Bio Solutions Canada Business Development Alderys France Director Head of Business Algenuity United Kingdom Development Senior Business Attraction Alliant Energy United States Manager Senior Economic Alliant Energy United States Development Manager Key Account Manager Alliant Energy United States CEO Altarum Institute France Research Chemical Amalgamated Research LLC United States Engineer Chemical Engineer -

513127 Arkansas Food Law Policy 3.2.Ps
University of Arkansas School of Law [email protected] $ (479) 575-7646 An Agricultural Law Research Article “Show-Me” No Rice Pharming: An Overview of the Introduction of and Opposition to Genetically Engineered Pharmaceutical Crops in the United States by Jillian S. Hishaw Originally published in JOURNAL OF FOOD LAW AND POLICY 3 J. FOOD L. & POL’Y 209 (2007) www.NationalAgLawCenter.org “SHOW-ME” NO RICE PHARMING: AN OVERVIEW OF THE INTRODUCTION OF AND OPPOSITION TO GENETICALLY ENGINEERED PHARMACEUTICAL CROPS IN THE UNITED STATES Jillian S. Hishaw* I. INTRODUCTION ......................................................................... 209 II. INEFFECTIVE REGULATORY CONTROLS .................................... 210 III. GENETICALLY MODIFIED CROP CONCERNS ............................. 214 A. Early Incidents ...................................................................... 215 B. The California Experience ..................................................... 218 C. The Missouri Welcome and Opposition ................................. 219 D. The Bayer Lawsuit ................................................................ 223 IV. CONCLUSION ............................................................................. 226 I. INTRODUCTION Farmers in California and Missouri have one thing in com- mon⎯opposition to the production of genetically modified (GM) “pharma” crops.1 A pharmaceutical crop, or “pharma” crop, is a plant that has been genetically altered so that it produces proteins which are used as drugs.2 Pharmaceutical companies can then harv- est the crop and isolate the proteins, which may be used to make * Jillian S. Hishaw received her J.D. in 2005 and LL.M in Agricultural Law in 2007, from the University of Arkansas School of Law, Fayetteville, Arkansas. Ms. Hishaw is a native of Kansas City, Missouri and currently works for the Missouri Department of Conservation. Ms. Hishaw would like to extend special thanks to Professor Susan Schneider for her devotion in helping her evolve into a more con- ceptual writer. -

STUDY on RISK ASSESSMENT: APPLICATION of ANNEX I of DECISION CP 9/13 to LIVING MODIFIED FISH Note by the Executive Secretary INTRODUCTION 1
CBD Distr. GENERAL CBD/CP/RA/AHTEG/2020/1/3 19 February 2020 ENGLISH ONLY AD HOC TECHNICAL EXPERT GROUP ON RISK ASSESSMENT AND RISK MANAGEMENT Montreal, Canada, 31 March to 3 April 2020 Item 3 of the provisional agenda* STUDY ON RISK ASSESSMENT: APPLICATION OF ANNEX I OF DECISION CP 9/13 TO LIVING MODIFIED FISH Note by the Executive Secretary INTRODUCTION 1. In decision CP-9/13, the Conference of the Parties serving as the meeting of the Parties to the Cartagena Protocol on Biosafety decided to establish the Ad Hoc Technical Expert Group (AHTEG) on Risk Assessment and Risk Management, which would work in accordance with the terms of reference contained in annex II to that decision. In the same decision, the Conference of the Parties serving as the meeting of the Parties to the Protocol requested the Executive Secretary to commission a study informing the application of annex I of the decision to (a) living modified organisms containing engineered gene drives and (b) living modified fish and present it to the online forum and the AHTEG. 2. Pursuant to the above, and with the financial support of the Government of the Netherlands, the Secretariat commissioned a study informing the application of annex I to living modified fish to facilitate the process referred to in paragraph 6 of decision CP-9/13. The study was presented to the Open-Ended Online Forum on Risk Assessment and Risk Management, which was held from 20 January to 1 February 2020, during which registered participants provided feedback and comments.1 3. -

Genetically Engineered Fish: an Unnecessary Risk to the Environment, Public Health and Fishing Communities
Issue brief Genetically engineered fish: An unnecessary risk to the environment, public health and fishing communities On November 19, 2015, the U.S. Food & Drug Administration announced its approval of the “AquAdvantage Salmon,” an Atlantic salmon that has been genetically engineered to supposedly be faster-growing than other farmed salmon. This is the first-ever genetically engineered animal allowed to enter the food supply by any regulatory agency in the world. At least 35 other species of genetically engineered fish are currently under development, including trout, tilapia, striped bass, flounder and other salmon species — all modified with genes from a variety of organisms, including other fish, coral, mice, bacteria and even humans.1 The FDA’s decision on the AquAdvantage genetically engineered salmon sets a precedent and could open the floodgates for other genetically engineered fish and animals (including cows, pigs and chickens) to enter the U.S. market. Genetically engineered salmon approved by FDA Despite insufficient food safety or environmental studies, the FDA announced its approval of the AquAdvantage Salmon, a genetically engineered Atlantic salmon produced by AquaBounty Technologies. The company originally submitted its application to the FDA in 2001 and the FDA announced in the summer of 2010 it was considering approval of this genetically engineered fish — the first genetically engineered animal intended for human consumption. In December 2012, the FDA released its draft Environmental Assessment of this genetically engineered salmon, and approved it in November 2015. This approval was made despite the 1.8 million people who sent letters to FDA opposing approval of the so-called “frankenfish,” and the 75 percent of respondents to a New York Times poll who said they would not eat genetically engineered salmon.2 The FDA said it would probably not require labeling of the fish; however Alaska, a top wild salmon producer, requires labeling of genetically engineered salmon and momentum is growing for GMO labeling in a number of states across the U.S. -

MONARCHS in PERIL HE MONARCH BUTTERFLY IS in SERIOUS TROUBLE —Their Numbers Have Tplummeted Over the Past Two Decades
MONARC HS IN PERIL HERBICIDE-RESISTANT CROPS AND THE DECLINE OF MONARCH BUTTERFLIES IN NORTH AMERICA EXECUTIVE SUMMARY FEBRUARY 2015 ABOUT CENTER FOR FOOD SAFETY CENTER FOR FOOD SAFETY (CFS) is a non-profit public interest and environmental advocacy membership organization established in 1997 for the purpose of challenging harmful food production technologies and promoting sustainable alternatives. CFS combines multiple tools and strategies in pur suing its goals, including litigation and legal petitions for rulemaking, legal support for various sustainable agriculture and food safety constituencies, as well as public education, grassroots organizing and media outreach. ACKNOWLEDGEMENTS Authors: BILL FREESE AND MARTHA CROUCH, P hD Executive Summary Contributing Writer: LARISSA WALKER Copy Editing: ABIGAIL SEILER, LARISSA WALKER, MADELEINE CARNEMARK Legal Consultant: GEORGE KIMBRELL Graphics: PATRICK RIGGS Design: HUMMINGBIRD DESIGN STUDIO Report Advisor: ANDREW KIMBRELL The authors are indebted to several reviewers, in particular Dr. Lincoln Brower, for their helpful comments and suggestions. CENTER FOR FOOD SAFETY MONARCHS IN PERIL HE MONARCH BUTTERFLY IS IN SERIOUS TROUBLE —their numbers have Tplummeted over the past two decades. The butterfly’s decline tracks the virtual eradication of its caterpillar’s chief food source—common milkweed—from Midwestern cropland. The demise of milkweed is due to intensive spraying of glyphosate herbicide on Monsanto’s Roundup Ready corn and soybeans that have been genetically engi - neered to withstand it. Monarchs are in imminent danger unless milkweed is restored to Midwestern crop fields. Milkweed cannot recover with continued heavy use of glyphosate on Roundup Ready crops. We face a historic choice: do we want to protect Monsanto or mon - archs? The threats to monarch survival will soon escalate, if new genetically engineered (GE) crops resistant to glyphosate and additional herbicides like 2,4-D and dicamba are introduced. -

Problems in Gene Therapy and Transgenesis
Problems in Gene Therapy and Transgenesis Prof. Dr. Oliver Kayser Technische Biochemie TU Dortmund 16.03.2015 Chapter 5 - Transgenesis and Gene 1 Therapy Producing transgenic animals Purpose: Production of heterolog proteins in transgenic animals Principle: Integration of heterolog DNA with selected genetic information in fertilised eggs Methods: Mikroinjektion (-) Retrovirus vectors (-) Genetically modified embryonic stem cells (++) 16.03.2015 Chapter 5 - Transgenesis and Gene Therapy 2 16.03.2015 Chapter 5 - Transgenesis and Gene Therapy 3 Dolly 16.03.2015 Chapter 5 - Transgenesis and Gene 4 Therapy Telomers 16.03.2015 Chapter 5 - Transgenesis and Gene 5 Therapy klausurrelevant 16.03.2015 Chapter 5 - Transgenesis and Gene Therapy 6 16.03.2015 Chapter 5 - Transgenesis and Gene Therapy 7 Advantage of sheeps and goats as transgenic animals • high milk production capacity (2-3 l/ day) • short gestation period and quick maturation • low capital investment • easy handling and breeding • high expression level of proteins (35g protein/ l milk) • easy downstream processing • ongoing supply of product is guaranteed by breeding 16.03.2015 Chapter 5 - Transgenesis and Gene 8 Therapy Drug safety • Genetic stability from animal to anmial • Variation in produktion und purity during lactation • Microbial, varial, and mykoplasm contamination • TSE, Scrapie • Age and general conditions of animals 16.03.2015 Chapter 5 - Transgenesis and Gene 9 Therapy Atryn • First recombinant therapeutic protein approved in 2009 • Anticoagulant for treatment of antithrombin -

Putting the Cartel Before the Horse ...And Farm, Seeds, Soil, Peasants, Etc
Communiqué www.etcgroup.org September 2013 No. 111 Putting the Cartel before the Horse ...and Farm, Seeds, Soil, Peasants, etc. Who Will Control Agricultural Inputs, 2013? In this Communiqué, ETC Group identifies the major corporate players that control industrial farm inputs. Together with our companion poster, Who will feed us? The industrial food chain or the peasant food web?, ETC Group aims to de-construct the myths surrounding the effectiveness of the industrial food system. Table of contents Introduction: 3 Messages 3 Seeds 6 Commercial Seeds 7 Pesticides and Fertilizers 10 Animal pharma 15 Livestock Genetics 17 Aquaculture Genetics Industry 26 Conclusions and Policy Recommendations 31 Introduction: 3 Messages ETC Group has been monitoring the power and global reach of agro-industrial corporations for several decades – including the increasingly consolidated control of agricultural inputs for the industrial food chain: proprietary seeds and livestock genetics, chemical pesticides and fertilizers and animal pharmaceu- ticals. Collectively, these inputs are the chemical and biological engines that drive industrial agriculture. This update documents the continuing concentration (surprise, surprise), but it also brings us to three conclusions important to both peasant producers and policymakers… 1. Cartels are commonplace. Regulators have lost sight of the well-accepted economic principle that the market is neither free nor healthy whenever 4 companies control more than 50% of sales in any commercial sector. In this report, we show that the 4 firms / 50% line in the sand has been substan- tially surpassed by all but the complex fertilizer sector. Four firms control 58.2% of seeds; 61.9% of agrochemicals; 24.3% of fertilizers; 53.4% of animal pharmaceuticals; and, in livestock genetics, 97% of poultry and two-thirds of swine and cattle research. -

Should Genetically Modified Salmon Be Part of the Human Food Chain? Todd Biddle Cumberland Valley High School July 2011
Should Genetically Modified salmon be part of the Human Food Chain? Todd Biddle Cumberland Valley High School July 2011 Authors note: This case study is based on a real and current political upheaval amongst the United States House of Representatives, the Senate, the FDA, the consumer, and AquaBountyTechnologies®. Photo courtesy of Living in a Modern World. Modernest. Photo courtesy of AquaBounty Technologies. Aqu Advantage Fish. AquaBounty September 2010. Technologies 2011. http://www.aquabounty.com/PressRoom/ http://blog.modernest.com/2010/09/21/an-upstream- swim-to-stop-ge-salmon/ Introduction: What is for dinner? The choices for our dinner plate continue to evolve due to modern technologies and new information about human health. One way the “undesirable” genetics of edible plants and animals can be changed over time is through the process of artificial selection, a form of selective breeding. Artificial selection involves agriculturists selecting plants and animals that have the most desirable traits for producing our food. These plants and animals become the parent stock of future generations. In recent years, creating genetically modified crops and now animals has become an alternative to artificial selection. Even though genetically modified crops have been part of the human diet since the 1990s, genetically modified foods are often met with criticism from global consumers (Note: the European Commission has received responses from some European countries who wish to ban genetically modified organisms). There is a lack of public trust in their safety and they flag a number of environmental concerns. Genetically modified salmon, AquAdvantage® Salmon, engineered by the company AquaBounty Technologies may become the first genetically modified animal protein approved by the Food and Drug Administration, FDA. -

Global GMO Litigation Case Type
Global GMO Litigation Analysis of Top Trends v-Fluence identified the one hundred most visible and influential legal cases centered on genetically modified organism. This case compilation is the product of research across two years of company market analysis and reporting, along with supplemental searches into U.S. legal databases and international legal news outlets. To better understand the legal environment for GMOs, v-Fluence has categorized cases by the nature of the disputes and their regions, as well as identified major stakeholders associated with ag-biotech litigation. Key findings are outlined below. Case Type GMO-related cases primarily fall into one of eight categories: • Toxic Tort/Personal Injury – Suits claiming GMO exposure causes harm. • Intellectual Property Claims – Emphasis on cases addressing farmer violations of corporate patents and royalty disputes • Traditional Common Law Tort-Based Claims – Disputes on cross-contamination, unintentional GM grain mixing • “All Natural” Claims – Actions against food makers and retailers alleging inaccurate or false advertising of “all natural” products containing GMOs. • Contract Related Claims – Corporate-grower seed saving disputes and contract violations • Other Claims – Often connected to biotech stakeholder activities – defamation • Regulatory Actions – Demands for regulatory enforcement and compliance and challenges to government approvals • Criminal Actions – Suits related to illegal GM seed sales, counterfeit GM seeds and seed theft Case Type Breakdown 13% 18% 2% 4% 5% 4% 16% 38% Common law tort claims "All Natural" Class Action Suits Regulatory Criminal Other Toxic Tort/Personal Injury Contract Related Claims Intellectual Property Regulatory-focused legal actions made up the largest share of the ag-biotech litigation landscape. These cases are largely driven by environmental and consumer health groups challenging government policy or lack of enforcement.