Transgenic Animals
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Salmon with a Side of Genetic Modification: the FDA's Approval of Aquadvantage Salmon and Why the Precautionary Principle Is E
Salmon with a Side of Genetic Modification: The FDA’s Approval of AquAdvantage Salmon and Why the Precautionary Principle is Essential for Biotechnology Regulation Kara M. Van Slyck* INTRODUCTION Over the last thirty years, once abundant wild salmon populations in the Pacific and Atlantic Oceans have declined to a mere fraction of their historic levels. As of 2016, salmon populations in Washington State’s Columbia River region are either failing to make any progress towards recovery or showing very little signs of improvement; Puget Sound salmon are only getting worse.1 In the Gulf of Maine, salmon populations dropped from five-hundred spawning adults in 1995 to less than fifty adults in 1999.2 Atlantic salmon, Salmo salar, were first designated as “endangered”3 in November 2000—the Fish and Wildlife Service (FWS) expanded the listing nine years later to include critical habitat along the coast of Maine as a result of little improvement to the population’s numbers.4 In the Pacific Ocean, FWS classified four significant salmon species as endangered for protection under the Endangered Species Act * Juris Doctor Candidate, Seattle University School of Law 2018. I would like to extend thanks to Professor Carmen G. Gonzalez for her valuable insight of the precautionary principle, her extensive knowledge on environmental regulations in the United States, and her unwavering support of this Note. 1. Governor’s Salmon Recovery Office, State of Salmon in Watersheds 2016, WASH. ST. RECREATION & CONSERVATION OFF., http://stateofsalmon.wa.gov/governors-report-2016/ [https:// perma.cc/6F3J-FKWE]. 2. Endangered and Threatened Species; Final Endangered Status for a Distinct Population Segment of Anadromous Atlantic Salmon (Salmo salar) in the Gulf of Maine, 50 C.F.R. -

Gene Use Restriction Technologies for Transgenic Plant Bioconfinement
Plant Biotechnology Journal (2013) 11, pp. 649–658 doi: 10.1111/pbi.12084 Review article Gene use restriction technologies for transgenic plant bioconfinement Yi Sang, Reginald J. Millwood and C. Neal Stewart Jr* Department of Plant Sciences, University of Tennessee, Knoxville, TN, USA Received 1 February 2013; Summary revised 3 April 2013; The advances of modern plant technologies, especially genetically modified crops, are considered accepted 9 April 2013. to be a substantial benefit to agriculture and society. However, so-called transgene escape *Correspondence (fax 1-865-974-6487; remains and is of environmental and regulatory concern. Genetic use restriction technologies email [email protected]) (GURTs) provide a possible solution to prevent transgene dispersal. Although GURTs were originally developed as a way for intellectual property protection (IPP), we believe their maximum benefit could be in the prevention of gene flow, that is, bioconfinement. This review describes the underlying signal transduction and components necessary to implement any GURT system. Keywords: transgenic plants, Furthermore, we review the similarities and differences between IPP- and bioconfinement- transgene escape, male sterility, oriented GURTs, discuss the GURTs’ design for impeding transgene escape and summarize embryo sterility, transgene deletion, recent advances. Lastly, we go beyond the state of the science to speculate on regulatory and gene flow. ecological effects of implementing GURTs for bioconfinement. Introduction proposed (Daniell, 2002; Gressel, 1999; Moon et al., 2011), including strategies for male sterility (Mariani et al., 1990), Transgenic crops have become an integral part of modern maternal inheritance (Daniell et al., 1998; Iamtham and Day, agriculture and have been increasingly adopted worldwide (James, 2000; Ruf et al., 2001), transgenic mitigation (Al-Ahmad et al., 2011). -

Recombinant DNA and Elements Utilizing Recombinant DNA Such As Plasmids and Viral Vectors, and the Application of Recombinant DNA Techniques in Molecular Biology
Fact Sheet Describing Recombinant DNA and Elements Utilizing Recombinant DNA Such as Plasmids and Viral Vectors, and the Application of Recombinant DNA Techniques in Molecular Biology Compiled and/or written by Amy B. Vento and David R. Gillum Office of Environmental Health and Safety University of New Hampshire June 3, 2002 Introduction Recombinant DNA (rDNA) has various definitions, ranging from very simple to strangely complex. The following are three examples of how recombinant DNA is defined: 1. A DNA molecule containing DNA originating from two or more sources. 2. DNA that has been artificially created. It is DNA from two or more sources that is incorporated into a single recombinant molecule. 3. According to the NIH guidelines, recombinant DNA are molecules constructed outside of living cells by joining natural or synthetic DNA segments to DNA molecules that can replicate in a living cell, or molecules that result from their replication. Description of rDNA Recombinant DNA, also known as in vitro recombination, is a technique involved in creating and purifying desired genes. Molecular cloning (i.e. gene cloning) involves creating recombinant DNA and introducing it into a host cell to be replicated. One of the basic strategies of molecular cloning is to move desired genes from a large, complex genome to a small, simple one. The process of in vitro recombination makes it possible to cut different strands of DNA, in vitro (outside the cell), with a restriction enzyme and join the DNA molecules together via complementary base pairing. Techniques Some of the molecular biology techniques utilized during recombinant DNA include: 1. -

Environmental Assessment for Aquadvantage ® Salmon
Environmental Assessment for AquAdvantage Salmon Environmental Assessment for AquAdvantage® Salmon An Atlantic salmon (Salmo salar L.) bearing a single copy of the stably integrated α-form of the opAFP-GHc2 gene construct at the α-locus in the EO-1α line Aqua Bounty Technologies, Inc. Submitted to the Center for Veterinary Medicine US Food and Drug Administration For Public Display August 25, 2010 Page 1 of 84 August 25, 2010 Environmental Assessment for AquAdvantage Salmon Table of Contents (Page 1 of 4) Title Page ............................................................................................................................. 1 Table of Contents ................................................................................................................. 2 List of Tables ....................................................................................................................... 6 List of Figures ...................................................................................................................... 6 List of Acronyms & Abbreviations ..................................................................................... 7 List of Definitions ................................................................................................................ 9 Summary ............................................................................................................................. 10 1.0 INTRODUCTION .................................................................................................. -

Genetic Engineering and Sustainable Crop Disease Management: Opportunities for Case-By-Case Decision-Making
sustainability Review Genetic Engineering and Sustainable Crop Disease Management: Opportunities for Case-by-Case Decision-Making Paul Vincelli Department of Plant Pathology, 207 Plant Science Building, College of Agriculture, Food and Environment, University of Kentucky, Lexington, KY 40546, USA; [email protected] Academic Editor: Sean Clark Received: 22 March 2016; Accepted: 13 May 2016; Published: 20 May 2016 Abstract: Genetic engineering (GE) offers an expanding array of strategies for enhancing disease resistance of crop plants in sustainable ways, including the potential for reduced pesticide usage. Certain GE applications involve transgenesis, in some cases creating a metabolic pathway novel to the GE crop. In other cases, only cisgenessis is employed. In yet other cases, engineered genetic changes can be so minimal as to be indistinguishable from natural mutations. Thus, GE crops vary substantially and should be evaluated for risks, benefits, and social considerations on a case-by-case basis. Deployment of GE traits should be with an eye towards long-term sustainability; several options are discussed. Selected risks and concerns of GE are also considered, along with genome editing, a technology that greatly expands the capacity of molecular biologists to make more precise and targeted genetic edits. While GE is merely a suite of tools to supplement other breeding techniques, if wisely used, certain GE tools and applications can contribute to sustainability goals. Keywords: biotechnology; GMO (genetically modified organism) 1. Introduction and Background Disease management practices can contribute to sustainability by protecting crop yields, maintaining and improving profitability for crop producers, reducing losses along the distribution chain, and reducing the negative environmental impacts of diseases and their management. -

A Short History of DNA Technology 1865 - Gregor Mendel the Father of Genetics
A Short History of DNA Technology 1865 - Gregor Mendel The Father of Genetics The Augustinian monastery in old Brno, Moravia 1865 - Gregor Mendel • Law of Segregation • Law of Independent Assortment • Law of Dominance 1865 1915 - T.H. Morgan Genetics of Drosophila • Short generation time • Easy to maintain • Only 4 pairs of chromosomes 1865 1915 - T.H. Morgan •Genes located on chromosomes •Sex-linked inheritance wild type mutant •Gene linkage 0 •Recombination long aristae short aristae •Genetic mapping gray black body 48.5 body (cross-over maps) 57.5 red eyes cinnabar eyes 67.0 normal wings vestigial wings 104.5 red eyes brown eyes 1865 1928 - Frederick Griffith “Rough” colonies “Smooth” colonies Transformation of Streptococcus pneumoniae Living Living Heat killed Heat killed S cells mixed S cells R cells S cells with living R cells capsule Living S cells in blood Bacterial sample from dead mouse Strain Injection Results 1865 Beadle & Tatum - 1941 One Gene - One Enzyme Hypothesis Neurospora crassa Ascus Ascospores placed X-rays Fruiting on complete body medium All grow Minimal + amino acids No growth Minimal Minimal + vitamins in mutants Fragments placed on minimal medium Minimal plus: Mutant deficient in enzyme that synthesizes arginine Cys Glu Arg Lys His 1865 Beadle & Tatum - 1941 Gene A Gene B Gene C Minimal Medium + Citruline + Arginine + Ornithine Wild type PrecursorEnz A OrnithineEnz B CitrulineEnz C Arginine Metabolic block Class I Precursor OrnithineEnz B CitrulineEnz C Arginine Mutants Class II Mutants PrecursorEnz A Ornithine -

Barriers to Adoption of GM Crops
Iowa State University Capstones, Theses and Creative Components Dissertations Fall 2021 Barriers to Adoption of GM Crops Madeline Esquivel Follow this and additional works at: https://lib.dr.iastate.edu/creativecomponents Part of the Agricultural Education Commons Recommended Citation Esquivel, Madeline, "Barriers to Adoption of GM Crops" (2021). Creative Components. 731. https://lib.dr.iastate.edu/creativecomponents/731 This Creative Component is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Creative Components by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. Barriers to Adoption of GM Crops By Madeline M. Esquivel A Creative Component submitted to the Graduate Faculty in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE Major: Plant Breeding Program of Study Committee: Walter Suza, Major Professor Thomas Lübberstedt Iowa State University Ames, Iowa 2021 1 Contents 1. Introduction ................................................................................................................................. 3 2. What is a Genetically Modified Organism?................................................................................ 9 2.1 The Development of Modern Varieties and Genetically Modified Crops .......................... 10 2.2 GM vs Traditional Breeding: How Are GM Crops Produced? -

UNITED STATES SECURITIES and EXCHANGE COMMISSION Washington, D.C
UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 10-K ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the fiscal year ended December 31, 2018 OR TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the transition period from to . Commission File Number: 001-36042 INTREXON CORPORATION (Exact name of registrant as specified in its charter) Virginia 26-0084895 (State or other jurisdiction of (I.R.S. Employer incorporation or organization) Identification Number) 20374 Seneca Meadows Parkway Germantown, Maryland 20876 (Address of principal executive offices) (Zip Code) Registrant's telephone number, including area code (301) 556-9900 Securities registered pursuant to Section 12(b) of the Act: Title of each class Name of each exchange on which registered Intrexon Corporation Common Stock, No Par Value Nasdaq Global Select Market Securities registered pursuant to Section 12(g) of the Act: None Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes No Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes No Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. -
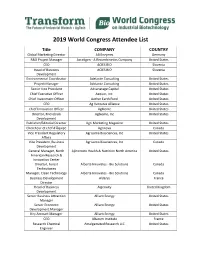
2019 World Congress Attendee List
2019 World Congress Attendee List Title COMPANY COUNTRY Global Marketing Director AB Enzymes Germany R&D Project Manager Acceligen - A Recombinetics Company United States CEO ACIES BIO Slovenia Head of Business ACIES BIO Slovenia Development Environmental Coordinator Adelante Consulting United States Project Manager Adelante Consulting United States Senior Vice President Advanatage Capital United States Chief Executive Officer Aequor, Inc. United States Chief Investment Officer Aether Earth Fund United States CEO Ag Ventures Alliance United States Chief Innovation Officer AgBiome United States Director, Microbials Agbiome, Inc United States Development Publisher/Editorial Director Agri Marketing Magazine United States Chercheur et chef d'Ãquipe Agrinova Canada Vice President Regulatory Agrisoma Biosciences, Inc United States Affairs Vice President, Business Agrisoma Biosciences, Inc Canada Development General Manager, North Ajinomoto Health & Nutrition North America United States American Research & Innovation Center Director, Forest Alberta Innovates - Bio Solutions Canada Technologies Manager, Clean Technology Alberta Innovates - Bio Solutions Canada Business Development Alderys France Director Head of Business Algenuity United Kingdom Development Senior Business Attraction Alliant Energy United States Manager Senior Economic Alliant Energy United States Development Manager Key Account Manager Alliant Energy United States CEO Altarum Institute France Research Chemical Amalgamated Research LLC United States Engineer Chemical Engineer -

World Resources Institute the Monsanto Company
World Resources Institute Sustainable Enterprise Program A program of the World Resources Institute The Monsanto Company: Quest for Sustainability (A) “Biotechnology represents a potentially sustainable For more than a decade, WRI's solution to the issue, not only of feeding people, but of providing Sustainable Enterprise Program (SEP) the economic growth that people are going to need to escape has harnessed the power of business to poverty…… [Biotechnology] poses the possibility of create profitable solutions to leapfrogging the industrial revolution and moving to a post- environment and development industrial society that is not only economically attractive, but challenges. BELL, a project of SEP, is also environmentally sustainable.i ” focused on working with managers and academics to make companies --Robert Shapiro, CEO, Monsanto Company more competitive by approaching social and environmental challenges as unmet market needs that provide Upon his promotion to CEO of chemical giant The business growth opportunities through Monsanto Company in 1995, Robert Shapiro became a vocal entrepreneurship, innovation, and champion of sustainable development and sought to redefine the organizational change. firm’s business strategy along principles of sustainability. Shapiro’s rhetoric was compelling. He captured analysts’ Permission to reprint this case is attention with the specter of mass hunger and environmental available at the BELL case store. degradation precipitated by rapid population growth and the Additional information on the Case -

STUDY on RISK ASSESSMENT: APPLICATION of ANNEX I of DECISION CP 9/13 to LIVING MODIFIED FISH Note by the Executive Secretary INTRODUCTION 1
CBD Distr. GENERAL CBD/CP/RA/AHTEG/2020/1/3 19 February 2020 ENGLISH ONLY AD HOC TECHNICAL EXPERT GROUP ON RISK ASSESSMENT AND RISK MANAGEMENT Montreal, Canada, 31 March to 3 April 2020 Item 3 of the provisional agenda* STUDY ON RISK ASSESSMENT: APPLICATION OF ANNEX I OF DECISION CP 9/13 TO LIVING MODIFIED FISH Note by the Executive Secretary INTRODUCTION 1. In decision CP-9/13, the Conference of the Parties serving as the meeting of the Parties to the Cartagena Protocol on Biosafety decided to establish the Ad Hoc Technical Expert Group (AHTEG) on Risk Assessment and Risk Management, which would work in accordance with the terms of reference contained in annex II to that decision. In the same decision, the Conference of the Parties serving as the meeting of the Parties to the Protocol requested the Executive Secretary to commission a study informing the application of annex I of the decision to (a) living modified organisms containing engineered gene drives and (b) living modified fish and present it to the online forum and the AHTEG. 2. Pursuant to the above, and with the financial support of the Government of the Netherlands, the Secretariat commissioned a study informing the application of annex I to living modified fish to facilitate the process referred to in paragraph 6 of decision CP-9/13. The study was presented to the Open-Ended Online Forum on Risk Assessment and Risk Management, which was held from 20 January to 1 February 2020, during which registered participants provided feedback and comments.1 3. -

1 Corporation Obtaining Approval, the Name of Its Representative, and the Address of Its Main Office Name: Bayer Crop Science K
Corporation obtaining approval, the name of its representative, and the address of its main office Name: Bayer Crop Science K.K. John Gray, President Address: Marunouchi Kitaguchi Building, 1-6-5, Marunouchi, Chiyoda-ku, Tokyo Approved Type 1 Use Regulation Name of the Type of Glufosinate herbicide tolerant, male sterile and fertility restored Living Modified oilseed rape (Modified bar, barnase, barstar, Brassica napus L.) Organism (MS1RF1, OECD UI :ACS-BNØØ4-7×ACS-BNØØ1-4) Content of the Type 1 Provision as food, provision as feed, processing, storage, Use of Living Modified transportation, disposal and acts incidental to them Organism Method of the Type 1 Use of Living Modified ― Organism 1 Outline of the Biological Diversity Risk Assessment Report I. Information collected prior to assessing Adverse Effect on Biological Diversity 1. Information concerning preparation of living modified organisms (1) Information concerning donor nucleic acid 1) Composition and origins of component elements Glufosinate herbicide tolerant and male sterile and fertility restored oilseed rape (modified bar, barnase, barstar, Brassica napus L., MS1RF1, OECD UI: ACS-BNØØ4-7 x ACS-BNØØ 1-4) (hereinafter referred to as “MS1RF1”) is a hybrid obtained by a cross between glufosinate herbicide tolerant and male sterile oilseed rape (modified bar, barnase, Brassica napus L., MS1, OECD UI: ACS-BNØØ4-7) (hereinafter referred to as “MS1”) and glufosinate herbicide tolerant and fertility restored oilseed rape (modified bar, barstar, Brassica napus L., RF1, OECD UI: ACS-BNØØ1-4) (hereinafter referred to as “RF1”). Composition of the donor nucleic acid that was used for the production of MS1 and RF1, the parent plants of MS1RF1, and the origins of component elements are shown in Table 1-1 and Table 1-2 respectively.