Swimming Upstream: the Eedn to Resolve Inconsistency in the FDA's Fishy Regulatory Scheme Kelsie Kelly
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Salmon with a Side of Genetic Modification: the FDA's Approval of Aquadvantage Salmon and Why the Precautionary Principle Is E
Salmon with a Side of Genetic Modification: The FDA’s Approval of AquAdvantage Salmon and Why the Precautionary Principle is Essential for Biotechnology Regulation Kara M. Van Slyck* INTRODUCTION Over the last thirty years, once abundant wild salmon populations in the Pacific and Atlantic Oceans have declined to a mere fraction of their historic levels. As of 2016, salmon populations in Washington State’s Columbia River region are either failing to make any progress towards recovery or showing very little signs of improvement; Puget Sound salmon are only getting worse.1 In the Gulf of Maine, salmon populations dropped from five-hundred spawning adults in 1995 to less than fifty adults in 1999.2 Atlantic salmon, Salmo salar, were first designated as “endangered”3 in November 2000—the Fish and Wildlife Service (FWS) expanded the listing nine years later to include critical habitat along the coast of Maine as a result of little improvement to the population’s numbers.4 In the Pacific Ocean, FWS classified four significant salmon species as endangered for protection under the Endangered Species Act * Juris Doctor Candidate, Seattle University School of Law 2018. I would like to extend thanks to Professor Carmen G. Gonzalez for her valuable insight of the precautionary principle, her extensive knowledge on environmental regulations in the United States, and her unwavering support of this Note. 1. Governor’s Salmon Recovery Office, State of Salmon in Watersheds 2016, WASH. ST. RECREATION & CONSERVATION OFF., http://stateofsalmon.wa.gov/governors-report-2016/ [https:// perma.cc/6F3J-FKWE]. 2. Endangered and Threatened Species; Final Endangered Status for a Distinct Population Segment of Anadromous Atlantic Salmon (Salmo salar) in the Gulf of Maine, 50 C.F.R. -

THE CASE for FIRST AMENDMENT PROTECTION of BIOART in 2000
FREEDOM TO EXPRESS A BIOLOGICAL CONSTRUCT: THE CASE FOR FIRST AMENDMENT PROTECTION OF BIOART KEITH SYVERSON INTRODUCTION In 2000, contemporary artist Eduardo Kac made international headlines by partnering with scientists at the Institut National de la Recherche Agronomique (INRA), France where together they genetically engineered an albino rabbit to express an enhanced green fluorescent protein (GFP)1 so that the rabbit would glow green when exposed to blue light.2 The entire project from its conception to the final display was intended as an artistic experiment to challenge society’s views on animal experimentation and the selective breeding of pets.3 This type of work that merges art and biology is called “BioArt.” According to Eduardo Kac, BioArt “employs one or more of the following approaches: (1) the coaching of biomaterials into specific inert shapes or behaviors; (2) the unusual or subversive use of biotech tools and processes; (3) the invention or transformation of living organisms with or without social environmental integration."4 BioArt has arguably existed for centuries.5 For example, some commentators have described the primitive methods of brewing beer in the Old Kingdom as form of BioArt.6 Similarly, the artificial selection involved in agriculture has been viewed by some as a form of 1 Frances Stracey, Bio-art: The Ethics Behind the Aesthetics, 10 NATURE REVIEWS: MOLECULAR CELL BIOLOGY 496, 499 (2009). 2 Id. 3 Id. 4 Eduardo Kac, Art That Looks You in the Eye: Hybrids, Clones, Mutants, Synthetics, and Transgenics, in SIGNS OF LIFE 1, 18 (Eduardo Kac ed., MIT Press, 2007). 5 See Edgar DaSilva, Art, Biotechnology and the Culture of Peace, 7 ELECTRONIC JOURNAL OF BIOTECHNOLOGY 130, 131-32 (2004) (arguing that the brewing reliefs in Egypt during the Old Kingdom from 2650 BC are an example of BioArt). -

Environmental Assessment for Aquadvantage ® Salmon
Environmental Assessment for AquAdvantage Salmon Environmental Assessment for AquAdvantage® Salmon An Atlantic salmon (Salmo salar L.) bearing a single copy of the stably integrated α-form of the opAFP-GHc2 gene construct at the α-locus in the EO-1α line Aqua Bounty Technologies, Inc. Submitted to the Center for Veterinary Medicine US Food and Drug Administration For Public Display August 25, 2010 Page 1 of 84 August 25, 2010 Environmental Assessment for AquAdvantage Salmon Table of Contents (Page 1 of 4) Title Page ............................................................................................................................. 1 Table of Contents ................................................................................................................. 2 List of Tables ....................................................................................................................... 6 List of Figures ...................................................................................................................... 6 List of Acronyms & Abbreviations ..................................................................................... 7 List of Definitions ................................................................................................................ 9 Summary ............................................................................................................................. 10 1.0 INTRODUCTION .................................................................................................. -

UNITED STATES SECURITIES and EXCHANGE COMMISSION Washington, D.C
UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 10-K ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the fiscal year ended December 31, 2018 OR TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the transition period from to . Commission File Number: 001-36042 INTREXON CORPORATION (Exact name of registrant as specified in its charter) Virginia 26-0084895 (State or other jurisdiction of (I.R.S. Employer incorporation or organization) Identification Number) 20374 Seneca Meadows Parkway Germantown, Maryland 20876 (Address of principal executive offices) (Zip Code) Registrant's telephone number, including area code (301) 556-9900 Securities registered pursuant to Section 12(b) of the Act: Title of each class Name of each exchange on which registered Intrexon Corporation Common Stock, No Par Value Nasdaq Global Select Market Securities registered pursuant to Section 12(g) of the Act: None Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes No Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes No Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. -
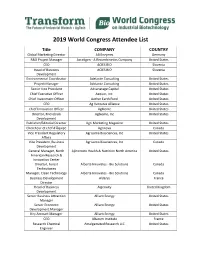
2019 World Congress Attendee List
2019 World Congress Attendee List Title COMPANY COUNTRY Global Marketing Director AB Enzymes Germany R&D Project Manager Acceligen - A Recombinetics Company United States CEO ACIES BIO Slovenia Head of Business ACIES BIO Slovenia Development Environmental Coordinator Adelante Consulting United States Project Manager Adelante Consulting United States Senior Vice President Advanatage Capital United States Chief Executive Officer Aequor, Inc. United States Chief Investment Officer Aether Earth Fund United States CEO Ag Ventures Alliance United States Chief Innovation Officer AgBiome United States Director, Microbials Agbiome, Inc United States Development Publisher/Editorial Director Agri Marketing Magazine United States Chercheur et chef d'Ãquipe Agrinova Canada Vice President Regulatory Agrisoma Biosciences, Inc United States Affairs Vice President, Business Agrisoma Biosciences, Inc Canada Development General Manager, North Ajinomoto Health & Nutrition North America United States American Research & Innovation Center Director, Forest Alberta Innovates - Bio Solutions Canada Technologies Manager, Clean Technology Alberta Innovates - Bio Solutions Canada Business Development Alderys France Director Head of Business Algenuity United Kingdom Development Senior Business Attraction Alliant Energy United States Manager Senior Economic Alliant Energy United States Development Manager Key Account Manager Alliant Energy United States CEO Altarum Institute France Research Chemical Amalgamated Research LLC United States Engineer Chemical Engineer -

Genetically Modified, Red Fluorescent Zebrafish: Detection, Crossing
Journal of Environmental Biotechnology Vol. 8, No. 2, 105–110, 2008 Original paper (regular paper) Genetically Modifi ed, Red Fluorescent Zebrafi sh: Detection, Crossing, Inheritance of Red Fluorescence, and Tolerance to Low Temperatures KIMIKO AMANUMA*, NOBUYOSHI NAKAJIMA, AKIKO H. HASHIMOTO and YASUNOBU AOKI National Institute for Environmental Studies, 16–2 Onogawa, Tsukuba, Ibaraki 305–8506, Japan * TEL: +81–(0)29–850–2390 FAX: +81–(0)29–850–2588 * E-mail: [email protected] (Received; 28 September, 2008/Accepted; 10 October, 2008) Under Cartagena Protocol domestic law, genetically modifi ed (GM) fi sh have not yet received permission to be imported or sold in Japan. However, it is anticipated that ornamental, fl uorescent GM fi sh will be imported and sold prior to any offi cial process for evaluating their eff ects on biological diversity as GM organisms (GMO). Therefore, we have devised a method for detecting GM fi sh that utilizes PCR for the detection of replication origins of plasmids that generally exist as transgenes integrated in the GMOs’ chromosomes. Using red fl uorescent zebrafi sh (rfZF), sold in Japan, and rpsL GM ZF established by us, we demonstrated the feasibility of this method for detecting GMO. Next, we examined the biological characteristics of the GM rfZF. They were able to cross with non-GM ZF; the red fl uorescence was inherited by their progeny according to expected outcomes, based on Mendelian genetics. Examination of the low temperature tolerance of rfZF and non-GM fi sh indicated that the lethal, minimum temperature was the same for both fi sh (5°C). -

Genome As a Tool of Genetic Engineering: Application in Plant and Plant Derived Medicine
International Journal of Biotech Trends and Technology (IJBTT) – Volume 8 Issue 1- Jan - March 2018 Genome as a Tool of Genetic Engineering: Application in Plant and Plant Derived Medicine A.B.M. Sharif Hossain1,2 Musamma M. Uddin2 1Department of Biology, Faculty of Science, Hail University, Hail, KSA 2Biotechnology Program, Institute of Biological Sciences, Faculty of Science, University of Malaya, 50603, Kuala Lumpur, Malaysia introduce point mutations. Genetically modified Abstract organism (GMO) is considered as an organism that The study was conducted from different is generated through genetic engineering. The first modern research data to review the innovative GMOs were bacteria in 1973, GM mice were generated in 1974 [4]. Insulin-producing bacteria latest technology in the genomics and its were commercialized in 1982 and genetically application in Agriculture, biomedicinae and modified food has been sold since 1994. Glofish, the plant derived medicine. Application of genome first GMO designed as a pet, was first sold in the in genetic engineering and molecular United States December in 2003 [4]. Genetic biotechnology have been exhibited well. engineering biotechnology has been applied in Genetically Modified Organism (GMO), numerous fields including agriculture, industrial Agrobacterium mediated recombination (T- biotechnology, and medicine. Enzymes used in DNA) and genetic engineering using molecular laundry detergent and medicines such as insulin and Biotechnology in plant, medicine and human growth hormone are now manufactured in biomedicine have been highlighted from GM cells, experimental GM cell lines and GM animals such as mice or zebra fish are being used for technology based different research data. research purposes, and genetically modified Moreover, molecular biotechnology in crops have been commercialized [4]. -

STUDY on RISK ASSESSMENT: APPLICATION of ANNEX I of DECISION CP 9/13 to LIVING MODIFIED FISH Note by the Executive Secretary INTRODUCTION 1
CBD Distr. GENERAL CBD/CP/RA/AHTEG/2020/1/3 19 February 2020 ENGLISH ONLY AD HOC TECHNICAL EXPERT GROUP ON RISK ASSESSMENT AND RISK MANAGEMENT Montreal, Canada, 31 March to 3 April 2020 Item 3 of the provisional agenda* STUDY ON RISK ASSESSMENT: APPLICATION OF ANNEX I OF DECISION CP 9/13 TO LIVING MODIFIED FISH Note by the Executive Secretary INTRODUCTION 1. In decision CP-9/13, the Conference of the Parties serving as the meeting of the Parties to the Cartagena Protocol on Biosafety decided to establish the Ad Hoc Technical Expert Group (AHTEG) on Risk Assessment and Risk Management, which would work in accordance with the terms of reference contained in annex II to that decision. In the same decision, the Conference of the Parties serving as the meeting of the Parties to the Protocol requested the Executive Secretary to commission a study informing the application of annex I of the decision to (a) living modified organisms containing engineered gene drives and (b) living modified fish and present it to the online forum and the AHTEG. 2. Pursuant to the above, and with the financial support of the Government of the Netherlands, the Secretariat commissioned a study informing the application of annex I to living modified fish to facilitate the process referred to in paragraph 6 of decision CP-9/13. The study was presented to the Open-Ended Online Forum on Risk Assessment and Risk Management, which was held from 20 January to 1 February 2020, during which registered participants provided feedback and comments.1 3. -

Genetically Engineered Fish: an Unnecessary Risk to the Environment, Public Health and Fishing Communities
Issue brief Genetically engineered fish: An unnecessary risk to the environment, public health and fishing communities On November 19, 2015, the U.S. Food & Drug Administration announced its approval of the “AquAdvantage Salmon,” an Atlantic salmon that has been genetically engineered to supposedly be faster-growing than other farmed salmon. This is the first-ever genetically engineered animal allowed to enter the food supply by any regulatory agency in the world. At least 35 other species of genetically engineered fish are currently under development, including trout, tilapia, striped bass, flounder and other salmon species — all modified with genes from a variety of organisms, including other fish, coral, mice, bacteria and even humans.1 The FDA’s decision on the AquAdvantage genetically engineered salmon sets a precedent and could open the floodgates for other genetically engineered fish and animals (including cows, pigs and chickens) to enter the U.S. market. Genetically engineered salmon approved by FDA Despite insufficient food safety or environmental studies, the FDA announced its approval of the AquAdvantage Salmon, a genetically engineered Atlantic salmon produced by AquaBounty Technologies. The company originally submitted its application to the FDA in 2001 and the FDA announced in the summer of 2010 it was considering approval of this genetically engineered fish — the first genetically engineered animal intended for human consumption. In December 2012, the FDA released its draft Environmental Assessment of this genetically engineered salmon, and approved it in November 2015. This approval was made despite the 1.8 million people who sent letters to FDA opposing approval of the so-called “frankenfish,” and the 75 percent of respondents to a New York Times poll who said they would not eat genetically engineered salmon.2 The FDA said it would probably not require labeling of the fish; however Alaska, a top wild salmon producer, requires labeling of genetically engineered salmon and momentum is growing for GMO labeling in a number of states across the U.S. -

Transgenic Fish Technology and Its Application in Aquaculture
* TRANSGENIC FISH TECHNOLOGY AND ITS APPLICATION IN AQUACULTURE Dr . G. B. CHAND Associate Professor Department of Zoology Aquatic Toxicology laboratory Patna University, Patna Email : [email protected] * Transgenic Fishes : Introduction . Organisms into which heterologous DNA (transgene) has been artificially introduced and integrated in their genomes are called transgenics. Genetically modified fish (GM fish) are organisms from the taxonomic clade which includes the classes Agnatha (jawless fish), Chondrichthyes (cartilaginous fish) and Osteichthyes (bony fish) whose genetic material (DNA) has been altered using genetic engineering techniques. In most cases, the aim is to introduce a new trait to the fish which does not occur naturally in the species, i.e. transgenesis. GM fish are used in scientific research and kept as pets. They are being developed as environmental pollutant sentinels and for use in aquaculture food production. In 2015, the Aqua-Advantage salmon was approved by the US Food and Drug Administration (FDA) for commercial production, sale and consumption, making it the first genetically modified animal to be approved for human consumption. Some GM fish that have been created have promoters driving an over-production of "all fish" growth hormone. This results in dramatic growth enhancement in several species, including salmonids, (Shao et al.,1992) carps (Robert et al.,2001) and Tilapias(Rahman et al.,2005; Hackett and Alvarez,2000). TRANSGENIC FISHES: INTRODUCTION . The first transgenic fish were produced in China in 1985( Dunham & Winn,2014). As of 2013, approximately 50 species of fish have been subject to genetic modification. This has resulted in more than 400 fish/trait combinations. Most of the modifications have been conducted on food species, such as Atlantic salmon (Salmo solar), tilapia (genus) and common carp (Cyprinus carpio).[Menozzi et al.,2013] What can transgenic technology offer? . -

Transgenic Animals
0( (-) 00 e_ ,Z5-1 Project Number: IQP — 43 — DSA — 9195 —5904 —0822 TRANSGENIC ANIMALS An Interactive Qualifying Project Report Submitted to the faculty of the WORCESTER POLYTECHNIC INSTITUTE In partial fulfillment of the requirements for the Degree of Bachelor of Science By Nicholas Far ey Scott LeBlanc Robert Redden Date: March 2, 2000 APPROVED: 1. transgenic 2. biotechnology 3. gene transfer Professor David S. Adams Project Advisor ABSTRACT This project investigated the use of transgenic animals in recent experiments and the effects of this novel technology on society. Using scientific journals and web resources, we examined the transgenic animals in existence, their uses, and the surrounding ethical and social issues. Recommendations were offered on which experiments involving transgenic animals should be continued and how to apply this new technology to the fields of science and medicine. ii TABLE OF CONTENTS SECTION PAGE Signature Page Abstract Table of Contents iii Executive Summary vii Project Objective xi List of Illustrations xii Introduction xiii 1. BACKGROUND AND HISTORY 1 1.1 Background Information 1 1.1.1 DNA 1 1.1.2 Replication and Expression 2 1.2 History of Transgenics 3 1.2.1 Prior to the First Transgenic Animal 3 1.2.2 The First Transgenic Animals 4 1.2.3 Further Experiments in the 1980's 5 1.2.4 Experiments Spanning into the Present 7 2. CURRENT METHODS 10 2.1 Superovulation 10 2.1.1 Choice of Parental Donor Strain 10 2.1.2 Hormone Cycle 11 2.1.3 Methods of Superovulation 13 iii 2.1.4 Flushing 14 2.2 Methods of Gene Transfer 17 2.2.1 Obtaining Recombinant DNA 17 2.2.2 Retroviral Vectors 19 2.2.3 Microinjection 20 2.2.4 Embryonic Stem Cells for Injections 22 2.2.5 Cultured Sperm Cells as Vectors 27 2.3 Transgenic Embryo Implantation 27 2.3.1 Embryo Inspection 27 2.3.2 Embryo Implantation Methods 28 2.3.3 Testing and Breeding 30 3. -

The Bio Revolution: Innovations Transforming and Our Societies, Economies, Lives
The Bio Revolution: Innovations transforming economies, societies, and our lives economies, societies, our and transforming Innovations Revolution: Bio The The Bio Revolution Innovations transforming economies, societies, and our lives May 2020 McKinsey Global Institute Since its founding in 1990, the McKinsey Global Institute (MGI) has sought to develop a deeper understanding of the evolving global economy. As the business and economics research arm of McKinsey & Company, MGI aims to help leaders in the commercial, public, and social sectors understand trends and forces shaping the global economy. MGI research combines the disciplines of economics and management, employing the analytical tools of economics with the insights of business leaders. Our “micro-to-macro” methodology examines microeconomic industry trends to better understand the broad macroeconomic forces affecting business strategy and public policy. MGI’s in-depth reports have covered more than 20 countries and 30 industries. Current research focuses on six themes: productivity and growth, natural resources, labor markets, the evolution of global financial markets, the economic impact of technology and innovation, and urbanization. Recent reports have assessed the digital economy, the impact of AI and automation on employment, physical climate risk, income inequal ity, the productivity puzzle, the economic benefits of tackling gender inequality, a new era of global competition, Chinese innovation, and digital and financial globalization. MGI is led by three McKinsey & Company senior partners: co-chairs James Manyika and Sven Smit, and director Jonathan Woetzel. Michael Chui, Susan Lund, Anu Madgavkar, Jan Mischke, Sree Ramaswamy, Jaana Remes, Jeongmin Seong, and Tilman Tacke are MGI partners, and Mekala Krishnan is an MGI senior fellow.