Notice of Convocation of the 15Th Term Annual Shareholders Meeting
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

View a Copy of This Licence, Visit Tivecommons.Org/Licenses/By/4.0
Katakami et al. Cardiovasc Diabetol (2020) 19:110 https://doi.org/10.1186/s12933-020-01079-4 Cardiovascular Diabetology ORIGINAL INVESTIGATION Open Access Tofoglifozin does not delay progression of carotid atherosclerosis in patients with type 2 diabetes: a prospective, randomized, open-label, parallel-group comparative study Naoto Katakami1,2* , Tomoya Mita3, Hidenori Yoshii4, Toshihiko Shiraiwa5, Tetsuyuki Yasuda6, Yosuke Okada7, Keiichi Torimoto7, Yutaka Umayahara8, Hideaki Kaneto9, Takeshi Osonoi10, Tsunehiko Yamamoto11, Nobuichi Kuribayashi12, Kazuhisa Maeda13, Hiroki Yokoyama14, Keisuke Kosugi15, Kentaro Ohtoshi16, Isao Hayashi17, Satoru Sumitani18, Mamiko Tsugawa19, Kayoko Ryomoto20, Hideki Taki21, Tadashi Nakamura22, Satoshi Kawashima23, Yasunori Sato24, Hirotaka Watada3 and Iichiro Shimomura1 on behalf of the UTOPIA study investigators Abstract Background: This study aimed to investigate the preventive efects of tofoglifozin, a selective sodium-glucose cotransporter 2 (SGLT2) inhibitor, on atherosclerosis progression in type 2 diabetes (T2DM) patients without apparent cardiovascular disease (CVD) by monitoring carotid intima-media thickness (IMT). Methods: This prospective, randomized, open-label, blinded-endpoint, multicenter, parallel-group, comparative study included 340 subjects with T2DM and no history of apparent CVD recruited at 24 clinical units. Subjects were randomly allocated to either the tofoglifozin treatment group (n 169) or conventional treatment group using drugs other than SGLT2 inhibitors (n 171). Primary outcomes were changes= in mean and maximum common carotid IMT measured by echography during= a 104-week treatment period. Results: In a mixed-efects model for repeated measures, the mean IMT of the common carotid artery (mean- IMT-CCA), along with the right and left maximum IMT of the CCA (max-IMT-CCA), signifcantly declined in both the tofoglifozin ( 0.132 mm, SE 0.007; 0.163 mm, SE 0.013; 0.170 mm, SE 0.020, respectively) and the control group ( 0.140 mm,− SE 0.006; 0.190 mm,− SE 0.012; 0.190 mm,− SE 0.020, respectively). -

CURRICULUM VITAE Kenneth J. Ruoff Contact Information
CURRICULUM VITAE Kenneth J. Ruoff Contact Information: Department of History Portland State University P.O. Box 751 Portland, OR 97207-0751 Tel. (503) 725-3991 Fax. (503) 725-3953 e-mail: [email protected] http://web.pdx.edu/~ruoffk/ Education Ph.D. 1997 Columbia University History M.Phil. 1993 Columbia University History M.A. 1991 Columbia University History B.A. 1989 Harvard College East Asian Studies Study of advanced Japanese, Inter-University Center (formerly known as the Stanford Center), Yokohama, Japan, 1993-1994 (this is a non-degree program). Awards Tim Garrison Faculty Award for Historical Research, Portland State University, 2020. For Japan's Imperial House in the Postwar Era, 1945-2019. Ambassador, Hokkaido University, 2019-present. Branford Price Millar Award for Faculty Excellence, Portland State University, Spring 2015. For excellence in the areas of research and teaching, in particular, but also for community service. Commendation, Consulate General of Japan, Portland, OR, December 2014. For enriching the cultural landscape of Portland through programs sponsored by the Center for Japanese Studies and for improving the understanding of Japan both through these programs and through scholarship. Frances Fuller Victor Award for General Nonfiction (best work of nonfiction by an Oregon author), Oregon Book Awards sponsored by the Literary Arts Organization, 2012. For Imperial Japan at Its Zenith: The Wartime Celebration of the Empire’s 2,600th Anniversary. Jirõ Osaragi Prize for Commentary (in Japanese, the Osaragi Jirõ rondanshõ), 2004, awarded by the Asahi Newspaper Company (Asahi Shinbun) for the best book in the social sciences published in Japan during the previous year (For Kokumin no tennõ; translation of The People’s Emperor). -

Ranking of Stocks by Market Capitalization(As of End of Mar.2020)
Ranking of Stocks by Market Capitalization(As of End of Mar.2020) 1st Section Rank Code Issue Market Capitalization \100mil. 1 7203 TOYOTA MOTOR CORPORATION 212,127 2 9437 NTT DOCOMO,INC. 112,630 3 6861 KEYENCE CORPORATION 84,709 4 6758 SONY CORPORATION 81,786 5 9984 SoftBank Group Corp. 79,162 6 9433 KDDI CORPORATION 75,136 7 4519 CHUGAI PHARMACEUTICAL CO.,LTD. 69,960 8 9432 NIPPON TELEGRAPH AND TELEPHONE CORPORATION 68,006 9 9434 SoftBank Corp. 65,799 10 7974 Nintendo Co.,Ltd. 54,787 11 8306 Mitsubishi UFJ Financial Group,Inc. 54,735 12 4568 DAIICHI SANKYO COMPANY,LIMITED 52,707 13 4502 Takeda Pharmaceutical Company Limited 52,146 14 4661 ORIENTAL LAND CO.,LTD. 50,261 15 6098 Recruit Holdings Co.,Ltd. 47,419 16 9983 FAST RETAILING CO.,LTD. 46,873 17 7182 JAPAN POST BANK Co.,Ltd. 44,865 18 4063 Shin-Etsu Chemical Co.,Ltd. 44,707 19 7267 HONDA MOTOR CO.,LTD. 44,017 20 4452 Kao Corporation 42,560 21 6367 DAIKIN INDUSTRIES,LTD. 38,603 22 6981 Murata Manufacturing Co.,Ltd. 36,980 23 8058 Mitsubishi Corporation 36,436 24 8316 Sumitomo Mitsui Financial Group,Inc. 36,018 25 9022 Central Japan Railway Company 35,679 26 8001 ITOCHU Corporation 35,541 27 8766 Tokio Marine Holdings,Inc. 35,145 28 7741 HOYA CORPORATION 34,808 29 6594 NIDEC CORPORATION 33,433 30 8035 Tokyo Electron Limited 32,000 31 3382 Seven & I Holdings Co.,Ltd. 31,699 32 7751 CANON INC. 31,463 33 8411 Mizuho Financial Group,Inc. -

Press Release
Press Release Company name: DAIICHI SANKYO COMPANY, LIMITED Representative: Sunao Manabe, Representative Director, President and CEO (Code no.: 4568, First Section, Tokyo Stock Exchange) Please address inquiries to Junichi Onuma, Vice President, Corporate Communications Department Telephone: +81-3-6225-1126 https://www.daiichisankyo.com Daiichi Sankyo Announces Transfer from Astellas Pharma of Three Products in Asia Tokyo, Japan (October 15, 2019) – Daiichi Sankyo Company, Limited (hereafter, Daiichi Sankyo) today announced that it agreed with Astellas Pharma Inc. (hereafter, Astellas Pharma) that Astellas Pharma local subsidiaries companies in six Asian countries will transfer three products to Daiichi Sankyo. The products to be transferred and the countries where they are sold are as follows. Product Korea China Taiwan Thailand Philippines Indonesia [generic name (brand name)] Ramosetron Antiemetic ○ ○ ○ ○ (Nasea) Nicardipine Anti- ○ ○ ○ (Perdipine) hypertensive Barnidipine Anti- ○ (Oldeca) hypertensive ○ indicate the countries where the products are sold The antiemetics are expected to have synergistic effects with mirogabalin and the cancer drugs that Daiichi Sankyo is currently developing in Asia, and the two antihypertensives are expected to effectively utilize Daiichi Sankyo’s current infrastructures in combination with its cardiovascular products, such as olmesartan and edoxaban. The total net sales of Astellas Pharma's three products in fiscal year 2018 were approximately 5.0 billion yen. 1 Daiichi Sankyo will take over the rights -

Long Diagnostic Delay with Unknown Transmission Route Inversely Correlates with the Subsequent Doubling Time of Coronavirus Disease 2019 in Japan, February–March 2020
International Journal of Environmental Research and Public Health Article Long Diagnostic Delay with Unknown Transmission Route Inversely Correlates with the Subsequent Doubling Time of Coronavirus Disease 2019 in Japan, February–March 2020 Tsuyoshi Ogata 1,* and Hideo Tanaka 2 1 Tsuchiura Public Health Center of Ibaraki Prefectural Government, Tsuchiura 300-0812, Japan 2 Fujiidera Public Health Center of Osaka Prefectural Government, Fujiidera 583-0024, Japan; [email protected] * Correspondence: [email protected] Abstract: Long diagnostic delays (LDDs) may decrease the effectiveness of patient isolation in reducing subsequent transmission of coronavirus disease 2019 (COVID-19). This study aims to investigate the correlation between the proportion of LDD of COVID-19 patients with unknown transmission routes and the subsequent doubling time. LDD was defined as the duration between COVID-19 symptom onset and confirmation ≥6 days. We investigated the geographic correlation between the LDD proportion among 369 confirmed COVID-19 patients with symptom onset between the 9th and 11th week and the subsequent doubling time for 717 patients in the 12th–13th week among the six prefectures. The doubling time on March 29 (the end of the 13th week) ranged from 4.67 days in Chiba to 22.2 days in Aichi. Using a Pearson’s product-moment correlation (p-value = 0.00182) and multiple regression analyses that were adjusted for sex and age (correlation coefficient −0.729, 95% confidence interval: −0.923–−0.535, p-value = 0.0179), the proportion of LDD for unknown Citation: Ogata, T.; Tanaka, H. Long exposure patients was correlated inversely with the base 10 logarithm of the subsequent doubling Diagnostic Delay with Unknown time. -
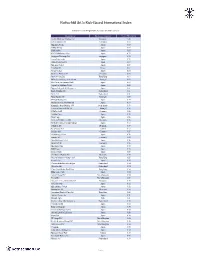
R&Co Risk-Based International Index – Weighting
Rothschild & Co Risk-Based International Index Indicative Index Weight Data as of June 30, 2021 on close Constituent Exchange Country Index Weight(%) Jardine Matheson Holdings Ltd Singapore 1.46 LEG Immobilien SE Germany 0.98 Ajinomoto Co Inc Japan 0.95 SoftBank Corp Japan 0.89 Shimano Inc Japan 0.85 FUJIFILM Holdings Corp Japan 0.73 Singapore Exchange Ltd Singapore 0.72 Japan Tobacco Inc Japan 0.72 Cellnex Telecom SA Spain 0.69 Nintendo Co Ltd Japan 0.69 Carrefour SA France 0.67 Nexon Co Ltd Japan 0.66 Deutsche Wohnen SE Germany 0.65 Bank of China Ltd Hong Kong 0.64 REN - Redes Energeticas Nacion Portugal 0.63 Pan Pacific International Hold Japan 0.63 Japan Post Holdings Co Ltd Japan 0.62 Nippon Telegraph & Telephone C Japan 0.61 Roche Holding AG Switzerland 0.61 Nestle SA Switzerland 0.61 Novo Nordisk A/S Denmark 0.59 ENEOS Holdings Inc Japan 0.59 Nomura Research Institute Ltd Japan 0.59 Koninklijke Ahold Delhaize NV Netherlands 0.59 Jeronimo Martins SGPS SA Portugal 0.58 HelloFresh SE Germany 0.58 Toshiba Corp Japan 0.58 Hoya Corp Japan 0.58 Siemens Healthineers AG Germany 0.58 MS&AD Insurance Group Holdings Japan 0.57 Coloplast A/S Denmark 0.57 Kerry Group PLC Ireland 0.57 Scout24 AG Germany 0.57 SG Holdings Co Ltd Japan 0.56 Symrise AG Germany 0.56 Nitori Holdings Co Ltd Japan 0.56 Beiersdorf AG Germany 0.55 Mitsubishi Corp Japan 0.55 KDDI Corp Japan 0.55 Sysmex Corp Japan 0.55 Chr Hansen Holding A/S Denmark 0.55 Ping An Insurance Group Co of Hong Kong 0.55 Eisai Co Ltd Japan 0.54 Chocoladefabriken Lindt & Spru Switzerland 0.54 Givaudan -

Association Between Variability in Body Mass Index and Development of Type 2 Diabetes: Panasonic Cohort Study
Epidemiology/Health services research Open access Original research BMJ Open Diab Res Care: first published as 10.1136/bmjdrc-2021-002123 on 22 April 2021. Downloaded from Association between variability in body mass index and development of type 2 diabetes: Panasonic cohort study Hiroshi Okada ,1 Masahide Hamaguchi ,2 Momoko Habu,1 Kazushiro Kurogi,3 Hiroaki Murata,4 Masato Ito,3 Michiaki Fukui2 To cite: Okada H, ABSTRACT Hamaguchi M, Habu M, Introduction Contrasting results have been reported for Significance of this study et al. Association between the association between the variability in body weight variability in body mass index and development of diabetes. In the present study, we What is already known about this subject? and development of type 2 evaluated the association between the variability in body ► Obesity or body weight gain accelerate the develop- diabetes: Panasonic cohort ment of diabetes. study. BMJ Open Diab Res Care mass index (BMI) and development of type 2 diabetes in 19 412 Japanese participants without obesity and without ► The association between the variability in body 2021;9:e002123. doi:10.1136/ weight and development of diabetes is controversial. bmjdrc-2021-002123 body weight gain or loss during the study period. Research design and methods We recorded body weight What are the new findings? of the participants consecutively each year in Panasonic ► The risk for developing diabetes increased by 11.1% Received 9 January 2021 Corporation, Osaka, Japan from 2008 to 2014 to evaluate Revised 26 February 2021 per 1% increase in coefficient of variation of body the variability of BMI. The participants with obesity (BMI mass index (BMI). -

Notice of the Ordinary General Meeting of Shareholders to Be Held on June 26, 2020
SONY CORPORATION Notice of the Ordinary General Meeting of Shareholders to be held on June 26, 2020 To the Registered Holders of American Depositary Receipts representing shares of Common Stock of Sony Corporation (the “Corporation”): The undersigned Depositary has received a notice that the Corporation has called an ordinary general meeting of shareholders to be held in Tokyo, Japan on June 26, 2020 (the “Meeting”) for the following purposes: MATTERS TO BE REPORTED: To receive reports on the business report, non-consolidated financial statements, consolidated financial statements, as well as audit reports on the consolidated financial statements by the Independent Auditors (certified public accountants) and the Audit Committee for the fiscal year ended March 31, 2020 (from April 1, 2019 to March 31, 2020) pursuant to the Companies Act of Japan. PROPOSALS TO BE ACTED UPON: 1. To amend a part of the Articles of Incorporation. 2. To elect 12 Directors. 3. To issue Stock Acquisition Rights for the purpose of granting stock options. EXPLANATION REGARDING THE SUBJECT MATTER OF THE MEETING MATTERS TO BE REPORTED: To receive reports on the business report, non-consolidated financial statements, consolidated financial statements, as well as audit reports on the consolidated financial statements by the Independent Auditors (certified public accountants) and the Audit Committee for the fiscal year ended March 31, 2020 (from April 1, 2019 to March 31, 2020). Note: The Consolidated Financial Statements pursuant to the Companies Act of Japan (Translation) are available on the Sony Investor Relations website. This document can be accessed at https://www.sony.net/SonyInfo/IR/stock/shareholders_meeting/Meeting103/ 1 PROPOSALS TO BE ACTED UPON: 1. -

Astellas Pharma Inc. 2-5-1, Nihonbashi-Honcho, Chuo-Ku, Tokyo 103-8411, Japan Astellas Pharma Inc
ANNUAL REPORT 2016 ANNUAL REPORT 2016 For the Year Ended March 31, 2016 Astellas Pharma Inc. 2-5-1, Nihonbashi-Honcho, Chuo-ku, Tokyo 103-8411, Japan http://www.astellas.com/en/ Astellas Pharma Inc. Please direct inquiries concerning Annual Report 2016 to: Astellas Pharma Inc. Corporate Communications TEL: +81-3-3244-3202 FAX: +81-3-5201-7473 This report is printed with environmentally friendly vegetable-based inks on FSCTM-certified paper made of wood sourced from responsibly managed forests, Issued in August 2016 using a waterless printing process. Printed in Japan Contents About Astellas 1 About Astellas 2 Business Philosophy Retracing Astellas’ Steps 3 Astellas Today 5 Contribute toward Health Financial and Non-Financial Highlights 7 Raison D'être Editorial Policy 9 Contribute toward improving the health of people through Innovative Drugs around the world through the provision of innovative 2 Our Strategy 10 and reliable pharmaceutical products 1 Astellas Value Creation Process 11 • To go beyond all others in exploring and tapping the potential CEO Message Astellas is concentrating on the 13 of the life sciences. CSR-Based Management 17 innovative drug business, where Business Environment 19 • To continue tackling new challenges and creating innovative it possesses key strengths. Strategic Plan 2015-2017 pharmaceutical products. 20 We are enhancing our ability to Financial Strategy 24 • To deliver quality products along with accurate information Management Structure 25 and retain solid credibility among customers. continuously develop innovative Interview with an Outside Director 27 drugs, in conjunction with • To support healthy living for people around the world. optimizing the allocation of • To continue shining on the global pharmaceutical field. -

National Survey Report of Japan 2019
Task 1 Strategic PV Analysis and Outreach National Survey Report of PV Power Applications in JAPAN 2019 Prepared by: Mitsuhiro YAMAZAKI, New Energy and Industrial Technology Development Organization (NEDO) Osamu IKKI, RTS Corporation PVPS Task 1 – National Survey Report of PV Power Applications in JAPANNational Survey Report of Japan 2019 What is IEA PVPS TCP? The International Energy Agency (IEA), founded in 1974, is an autonomous body within the framework of the Organization for Economic Cooperation and Development (OECD). The Technology Collaboration Programme (TCP) was created with a belief that the future of energy security and sustainability starts with global collaboration. The programme is made up of 6.000 experts across government, academia, and industry dedicated to advancing common research and the application of specific energy technologies . The IEA Photovoltaic Power Systems Programme (IEA PVPS) is one of the TCP’s within the IEA and was established in 1993. The mission of the programme is to “enhance the international collaborative efforts which facilitate the role of photovoltaic sol ar energy as a cornerstone in the transition to sustainable energy systems.” In order to achieve this, the Programme’s participants have undertaken a variety of joint research projects in PV power systems applications. The overall programme is headed by an Execu tive Committee, comprised of one delegate from each country or organisation member, which designates distinct ‘Tasks,’ that may be research projects or activity areas. The IEA PVPS participating countries are Australia, Austria, Belgium, Canada, Chile, China, Denmark, Finland, France, Germany, Israel, Italy, Japan, Korea, Malaysia, Mexico, Morocco, the Netherlands, Norway, Portugal, South Africa, Spain, Sweden, Switzerland, Thailand, Turkey, and the United States of America. -

INTA Corporate Member List
Corporate Members Name City State Country 100x Group Hong Kong Hong Kong 1661, Inc. Los Angeles California United States 1-800-Flowers.com, Inc. Carle Place New York United States 3M China Ltd. Shanghai Shanghai China 3M Company Saint Paul Minnesota United States 3M Deutschland GmbH Neuss Germany 3M India Ltd. Bangalore Karnataka India 3M Innovation Singapore Pte Ltd Singapore Singapore 3M KCI San Antonio Texas United States 3M United Kingdom PLC Bracknell Bracknell Forest United Kingdom 7-Eleven, Inc. Irving Texas United States AB Electrolux Stockholm Stockholms län Sweden Västra Götalands AB Volvo Göteborg län Sweden ABB ASEA Brown Boveri Ltd. Zürich Zürich Switzerland Abbott St.Paul Minnesota United States Abbott Laboratories Abbott Park Illinois United States Abbott Products Operations AG Allschwil Switzerland AbbVie Inc. Irvine California United States AbbVie Inc. North Chicago Illinois United States Abercrombie & Fitch Co. New Albany Ohio United States ABRO Industries, Inc. South Bend Indiana United States ACCO Brands Corporation Lake Zurich Illinois United States Activision Blizzard UK Ltd. Slough United Kingdom Activision Blizzard, Inc. Santa Monica California United States Activision Computer Technology Co. Ltd. Shanghai Shanghai China Acushnet Company Fairhaven Massachusetts United States Aderans America Holdings, Inc. Beverly Hills California United States adidas Group Panama Panamá Panama Adidas Group, India Gurgaon Haryana India adidas International Marketing BV Amsterdam Netherlands adidas International, Inc. Portland Oregon United States ADOBE INC. San Jose California United States ADOBE INC. Tokyo Japan Aetna Inc. Hartford Connecticut United States Agilent Technologies, Inc. Santa Clara California United States Ahold Delhaize Licensing Sarl Genève Genève Switzerland AIDA Cruises Rostock Germany Aigle International S.A. -

Astellas Pharma US, Inc. Corporate Integrity Agreement
CORPORATE INTEGRITY AGREEMENT BETWEEN THE OFFICE OF INSPECTOR GENERAL OF THE DEPARTMENT OF HEALTH AND HUMAN SERVICES AND ASTELLAS PHARMA US, INC. I. PREAMBLE Astellas Pharma US Inc. (Astellas) hereby enters into this Corporate Integrity Agreement (CIA) with the Office of Inspector General (OIG) of the United States Department of Health and Human Services (HHS) to promote compliance with the statutes, regulations, and written directives of Medicare, Medicaid, and all other Federal health care programs (as defined in 42 U.S.C. § 1320a-7b(f)) (Federal health care program requirements). Contemporaneously with this CIA, Astellas is entering into a Settlement Agreement with the United States. Prior to the Effective Date, Astellas established a compliance program that Astellas represents addresses all seven elements of an effective compliance program and that is designed to address compliance with Federal health care program requirements (Compliance Program). Astellas shall continue the Compliance Program throughout the term of the CIA and shall do so in accordance with the terms set forth below. Astellas may modify the Compliance Program as appropriate. However, at a minimum, Astellas shall ensure that during the term of this CIA, it shall maintain a compliance program to comply with the obligations set forth in this CIA. II. TERM AND SCOPE OF THE CIA A. The period of the compliance obligations assumed by Astellas under this CIA shall be five years from the effective date of this CIA. The “Effective Date” shall be the date on which the final signatory of this CIA executes this CIA. Each one-year period, beginning with the one-year period following the Effective Date, shall be referred to as a “Reporting Period.” Corporate Integrity Agreement Astellas Pharma US, Inc.