United Stat Apr
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

OFR Staff Plan
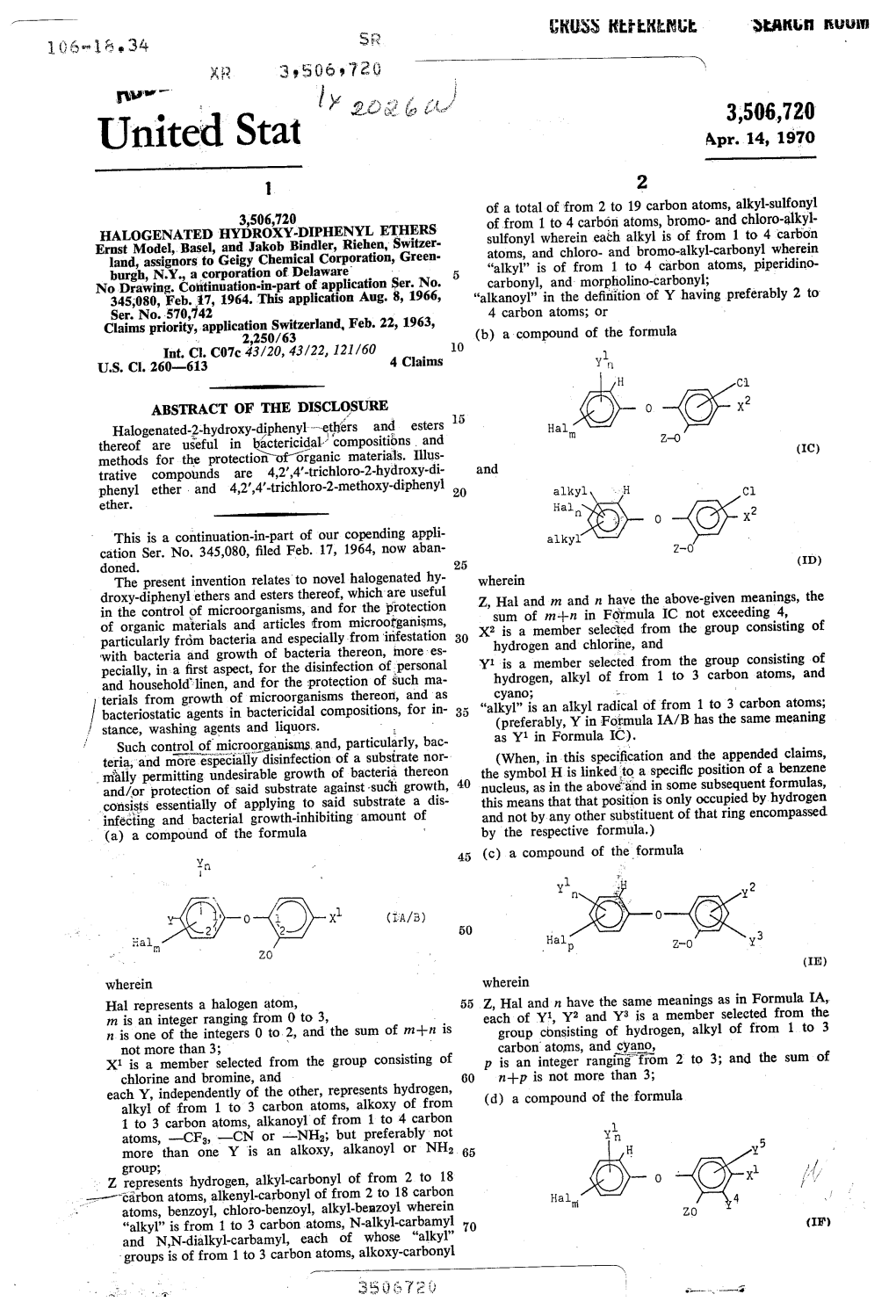
Staff Briefing Package Project Plan: Organohalogen Flame Retardant Chemicals Assessment July 1, 2020 CPSC Consumer Hotline and General Information: 1-800-638-CPSC (2772) CPSC's Web Site: http://www.cpsc.gov THIS DOCUMENT HAS NOT BEEN REVIEWED CLEARED FOR PUBLIC RELEASE OR ACCEPTED BY THE COMMISSION UNDER CPSA 6(b)(1) Acknowledgments The preparation, writing, and review of this report was supported by a team of staff. We acknowledge and thank team members for their significant contributions. Michael Babich, Ph.D., Directorate for Health Sciences Charles Bevington, M.P.H., Directorate for Health Sciences Xinrong Chen, Ph.D., D.A.B.T., Directorate for Health Sciences Eric Hooker, M.S., D.A.B.T., Directorate for Health Sciences Cynthia Gillham, M.S., Directorate for Economic Analysis John Gordon, Ph.D., Directorate for Health Sciences Kristina Hatlelid, Ph.D., M.P.H., Directorate for Health Sciences Barbara Little, Attorney, Office of the General Counsel Joanna Matheson, Ph.D., Directorate for Health Sciences ii THIS DOCUMENT HAS NOT BEEN REVIEWED CLEARED FOR PUBLIC RELEASE OR ACCEPTED BY THE COMMISSION UNDER CPSA 6(b)(1) Table of Contents Briefing Memo ............................................................................................................................... iv 1. Executive summary .............................................................................................................. 5 2. Introduction ......................................................................................................................... -

The Ozonolysis of Phenyl Grignard Reagent
University of Montana ScholarWorks at University of Montana Graduate Student Theses, Dissertations, & Professional Papers Graduate School 1971 The ozonolysis of phenyl Grignard reagent Gale Manning Sherrodd The University of Montana Follow this and additional works at: https://scholarworks.umt.edu/etd Let us know how access to this document benefits ou.y Recommended Citation Sherrodd, Gale Manning, "The ozonolysis of phenyl Grignard reagent" (1971). Graduate Student Theses, Dissertations, & Professional Papers. 8297. https://scholarworks.umt.edu/etd/8297 This Thesis is brought to you for free and open access by the Graduate School at ScholarWorks at University of Montana. It has been accepted for inclusion in Graduate Student Theses, Dissertations, & Professional Papers by an authorized administrator of ScholarWorks at University of Montana. For more information, please contact [email protected]. THE OZONOLYSIS OF PHENYL GRIGNARD REAGENT By Gale M. Sherrodd B.S., Rocky Mountain College, I969 Presented in partial fulfillment of the requirements for the degree of Master of Arts for Teachers UNIVERSITY OF MONTANA 1971 Approved by: Chairman, Board of Examiners De^ , Graduate *School / n ? / Date Reproduced with permission of the copyright owner. Further reproduction prohibited without permission. UMI Number: EP39098 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a complete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. UMT DiMMtstion PuWiahing UMI EP39098 Published by ProQuest LLC (2013). Copyright in the Dissertation held by the Author. -

Polybrominated Diphenyl Ethers (Pbdes)
America’s Children and the Environment, Third Edition DRAFT Indicators Biomonitoring: Polybrominated diphenyl ethers (PBDEs) EPA is preparing the third edition of America’s Children and the Environment (ACE3), following the previous editions published in December 2000 and February 2003. ACE is EPA’s compilation of children’s environmental health indicators and related information, drawing on the best national data sources available for characterizing important aspects of the relationship between environmental contaminants and children’s health. ACE includes four sections: Environments and Contaminants, Biomonitoring, Health, and Special Features. EPA has prepared draft indicator documents for ACE3 representing 23 children's environmental health topics and presenting a total of 42 proposed children's environmental health indicators. This document presents the draft text, indicator, and documentation for the PBDEs topic in the Biomonitoring section. THIS INFORMATION IS DISTRIBUTED SOLELY FOR THE PURPOSE OF PRE- DISSEMINATION PEER REVIEW UNDER APPLICABLE INFORMATION QUALITY GUIDELINES. IT HAS NOT BEEN FORMALLY DISSEMINATED BY EPA. IT DOES NOT REPRESENT AND SHOULD NOT BE CONSTRUED TO REPRESENT ANY AGENCY DETERMINATION OR POLICY. For more information on America’s Children and the Environment, please visit www.epa.gov/ace. For instructions on how to submit comments on the draft ACE3 indicators, please visit www.epa.gov/ace/ace3drafts/. March 2011 DRAFT: DO NOT QUOTE OR CITE Biomonitoring: Polybrominated Diphenyl Ethers (PBDEs) 1 Polybrominated Diphenyl Ethers (PBDEs) 2 3 Polybrominated diphenyl ethers (PBDEs) are a group of brominated flame retardant chemicals 4 that have been incorporated into a variety of manufactured products, including foam cushioning 5 used in furniture and plastics used in televisions and computers. -

Exposure and Use Assessment for Five PBT Chemicals
EPA Document # EPA-740-R1-8002 June 2018 United States Office of Chemical Safety and Environmental Protection Agency Pollution Prevention Exposure and Use Assessment of Five Persistent, Bioaccumulative and Toxic Chemicals Peer Review Draft June 2018 Contents TABLES ................................................................................................................................................................... 7 FIGURES ................................................................................................................................................................. 7 1. EXECUTIVE SUMMARY ................................................................................................................................ 15 2. BACKGROUND ............................................................................................................................................. 15 3. APPROACH .................................................................................................................................................. 17 4. DECABROMODIPHENYL ETHER (DECABDE) .................................................................................................. 21 4.1. Chemistry and Physical-Chemical Properties ................................................................................ 21 4.2. Uses ................................................................................................................................................ 21 4.3. Characterization of Expected Environmental Partitioning -

Certificate of Analysis
National Institute of Standards and Technology Certificate of Analysis Standard Reference Material 2257 PBDE Congeners in 2,2,4-Trimethylpentane This Standard Reference Material (SRM) is a solution of 38 polybrominated diphenyl ether (PBDE) congeners in 2,2,4-trimethylpentane. This SRM is intended primarily for use in the calibration of chromatographic instrumentation used for the determination of PBDE congeners. A unit of SRM 2257 consists of five 2 mL ampoules, each containing approximately 1.2 mL of solution. Certified Concentrations of Constituents: The certified concentration values and estimated uncertainties for the select constituents, expressed as mass fractions, are given in Table 1 along with the Chemical Abstract Service (CAS) Registry Numbers. The certified concentration values are based on results obtained from the gravimetric preparation of this solution and from the analytical results determined by using gas chromatography. A NIST certified value is a value for which NIST has the highest confidence in its accuracy in that all known or suspected sources of bias have been investigated or accounted for by NIST [1]. The measurands are the total concentrations of analytes shown on Table 1. Values are metrologically traceable to the International System of Units (SI) derived unit for mass fraction expressed as micrograms per gram. Expiration of Certification: The certification of SRM 2257 is valid, within the measurement uncertainty specified, until 31 July 2028, provided the SRM is handled and stored in accordance with the instructions given in this certificate (see “Instructions for Handling, Storage, and Use”). The certification is nullified if the SRM is damaged, contaminated, or modified. -

And Dechlorane Plus (DP) Flame Retardants in Marine Mussels (Mytilus Galloprovincialis) Enrique Barón, Awantha Dissanayake, Judit Vila, Charlotte Crowther, James W
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Plymouth Marine Science Electronic Archive (PlyMSEA) Subscriber access provided by UNIV OF PLYMOUTH Article Evaluation of the genotoxic and physiological effects of decabromodiphenyl ether (BDE-209) and dechlorane plus (DP) flame retardants in marine mussels (Mytilus galloprovincialis) Enrique Barón, Awantha Dissanayake, Judit Vila, Charlotte Crowther, James W. Readman, Awadhesh Jha, Ethel Eljarrat, and Damia Barcelo Environ. Sci. Technol., Just Accepted Manuscript • DOI: 10.1021/acs.est.5b05814 • Publication Date (Web): 01 Feb 2016 Downloaded from http://pubs.acs.org on February 4, 2016 Just Accepted “Just Accepted” manuscripts have been peer-reviewed and accepted for publication. They are posted online prior to technical editing, formatting for publication and author proofing. The American Chemical Society provides “Just Accepted” as a free service to the research community to expedite the dissemination of scientific material as soon as possible after acceptance. “Just Accepted” manuscripts appear in full in PDF format accompanied by an HTML abstract. “Just Accepted” manuscripts have been fully peer reviewed, but should not be considered the official version of record. They are accessible to all readers and citable by the Digital Object Identifier (DOI®). “Just Accepted” is an optional service offered to authors. Therefore, the “Just Accepted” Web site may not include all articles that will be published in the journal. After a manuscript is technically edited and formatted, it will be removed from the “Just Accepted” Web site and published as an ASAP article. Note that technical editing may introduce minor changes to the manuscript text and/or graphics which could affect content, and all legal disclaimers and ethical guidelines that apply to the journal pertain. -

Synthesis of Organobromines As a Tool for Their Characterisation and Environmental Occurrence Assessment
Synthesis of organobromines as a tool for their characterisation and environmental occurrence assessment Andreas Rydén Department of Materials and Environmental Chemistry Stockholm University Stockholm 2013 i Doctoral Thesis 2013 Department of Materials and Environmental Chemistry Stockholm University SE-106 91 Stockholm Sweden Abstract Polybrominated diphenyl ethers (PBDEs) have been intensively used as flame retardants (FRs) and have become ubiquitous environmental pollutants. PBDEs form hydroxylated PBDEs (OH-PBDEs) as metabolites. Further, some OH-PBDEs and methoxy-PBDEs (MeO-PBDEs) are natural products. These are all compounds of environmental and health concern and it is therefore important to confirm their identity and to assess their environmental levels and toxicities. Hence, it is vital to obtain authentic reference standards of individual PBDEs and OH/MeO-PBDEs. The thesis main aim was to develop synthesis methods of congener specific PBDEs, OH- and MeO-PBDEs. The second aim was to identify and quantify PBDEs, OH- and MeO-PBDEs in environmental samples. The third was to propose an abbreviation system for FRs. O-Arylation of brominated phenols, using either symmetrical or unsymmetrical brominated diphenyliodonium salts, was selected for synthesis of PBDEs and OH- /MeO-PBDEs. A total of 16 MeO-PBDEs, 11 OH-PBDEs, 1 diMeO-PBDE and 1 EtO-MeO-PBDE were synthesised. Three novel unsymmetrical diaryliodonium triflates were synthesised and used in synthesis. Optimisations were made to construct a reliable general method for congener specific PBDE synthesis, which was used in the synthesis of 8 representative PBDE congeners. The products were generally characterised by electron ionisation mass spectrometry (EIMS) and nuclear magnetic resonance (NMR) spectroscopy. -

Diphenyl and Phenyl Ether Method Number: PV2022 Target Concentration
Diphenyl and Phenyl ether Method number: PV2022 Target concentration: Diphenyl 0.2 ppm (1 mg/m3) OSHA TWA PEL Phenyl ether 1 ppm (7 mg/m3) OSHA TWA PEL Phenyl ether-Biphenyl mixture 1 ppm (7 mg/m3) OSHA TWA PEL Procedure: Samples are collected by drawing a known volume of air through an XAD-7 tube. Samples are desorbed with carbon disulfide and analyzed by gas chromatography with a flame ionization detector (GC-FID). Air volume and sampling rate studied: 100 minutes at 0.2 Lpm (20 L) Status of method: Partially Validated method. This method has been only partially evaluated and is presented for information and trial use. May 1988 Mary E. Eide SOLVENTS BRANCH OSHA Salt Lake Technical Center Salt Lake City UT 84115-1802 1 of 11 1 General Discussion 1.1 Background 1.1.1 History of procedure The OSHA Laboratory recently received air samples collected on XAD-7 sample tubes requesting analysis for diphenyl and phenyl ether. Desorption with carbon disulfide was tried and found to give desorption efficiencies of 99.7% for diphenyl and 98.6% for phenyl ether. Storage and retention efficiencies were similar. 1.1.2 Potential workplace exposure (Ref 5.1, 5.2, and 5.3) Diphenyl-phenyl ether mixture is used as a heat transfer agent. Diphenyl is also used as a fungistat for citrus fruit on wrappers and cardboard boxes and in organic synthesis. Phenyl ether is used in perfumes, soaps, and organic synthesis. 1.1.3 Toxic Effects (This section is for information purposes and should not be taken as the basis for OSHA policy.) (Ref 5.1 and 5.3) The mixture of diphenyl and phenyl ether is mildly irritating to skin, eyes, and mucous membranes, and can cause nausea. -

Probing the Lignin Disassembly Pathways with Modified Catalysts Based on Cu-Doped Porous Metal Oxides
Probing the lignin disassembly pathways with modified catalysts based on Cu-doped porous metal oxides. Megan Chui,a,§ Gustavo Metzker,a,b,§,* Christopher M. Bernt,a Anthony T. Tran,a Antonio C.B. Burtoloso,b Peter C. Ford.a,* a Department of Chemistry and Biochemistry, University of California, Santa Barbara, Santa Barbara, California, 93106-9510 United States. b Chemistry Institute at São Carlos, University of São Paulo, São Carlos, SP 13560-970 Brazil § These authors contributed equally. * Corresponding Authors: <[email protected]>; <[email protected]>. Abstract: Described are the selectivities observed for reactions of lignin model compounds with modifications of the copper-doped porous metal oXide (CuPMO) system previously shown to be a catalyst for lignin disassembly in super-critical methanol (Matson et al, J. Amer. Chem. Soc. 2011, 133, 14090-14097). The models studied are benzyl phenyl ether, 2- phenylethyl phenyl ether, diphenyl ether, biphenyl and 2,3 dihydrobenzofuran, which are respective mimetics of the α-O-4, β-O-4, 4-O-5, 5-5 and β-5 linkages characteristic of lignin. Also briefly investigated as a substrate is poplar organosolv lignin. The catalyst modifications included added samarium(III) (both homogeneous and heterogeneous) or formic acid. The highest activity for the hydrogenolysis of aryl ether linkages was noted for catalysts With Sm(III) incorporated into the solid matriX of the PMO structure. In contrast, simply adding Sm3+ salts to the solution suppressed the hydrogenolysis activity. Added formic acid suppressed aryl ether hydrogenolysis, presumably by neutralizing base sites on the PMO surface but at the same time improved the selectivity toWard aromatic products. -

Polybrominated Diphenyl Ethers (Pbdes)
Canadian Environmental Protection Act, 1999 Ecological Screening Assessment Report on Polybrominated Diphenyl Ethers (PBDEs) June 2006 Environment Canada where x + y = 1 to 10 Figure 1. PBDE structure Introduction The Canadian Environmental Protection Act, 1999 (CEPA 1999) requires the Minister of the Environment and the Minister of Health to conduct screening assessments of substances that meet the categorization criteria set out in the Act and Regulations to determine, in an expeditious manner, whether substances present or may present a risk to the environment or to human health. Based on the results of a screening assessment, the Ministers can propose taking no further action with respect to the substance, adding the substance to the Priority Substances List (PSL) for further assessment, or recommending that the substance be added to Schedule 1 of CEPA 1999 and, where applicable, the implementation of virtual elimination. A screening assessment involves an analysis of a substance using conservative assumptions to determine whether the substance meets the criteria as defined in section 64 of CEPA 1999. This ecological screening assessment examines various supporting information and develops conclusions based on a weight of evidence approach as required under Section 76.1 of CEPA 1999. The screening assessment does not represent an exhaustive review of all available data; rather, it presents the most critical studies and lines of evidence supporting the conclusions. One line of evidence includes consideration of risk quotients to identify potential for ecological effects. However, other concerns that affect current or potential risk, such as persistence, bioaccumulation, chemical transformation and trends in ambient concentrations, are also examined in this report. -

Polybrominated Diphenyl Ethers (Pbdes)
DRAFT FOR ÉBAUCHE POUR PUBLIC COMMENTS COMMENTAIRES DU PUBLIC Canadian Environmental Protection Act, 1999 Environmental Screening Assessment Report on Polybrominated Diphenyl Ethers (PBDEs) February 2004 Environment Canada where x + y = 1 to 10 Figure 1. PBDE structure Introduction The Canadian Environmental Protection Act, 1999 (CEPA 1999) requires the Minister of the Environment and the Minister of Health to conduct screening assessments of substances that meet the categorization criteria set out in the Act and Regulations to determine, in an expeditious manner, whether substances present or may present a risk to the environment or to human health. Based on the results of a screening assessment, the Ministers can propose taking no further action with respect to the substance, adding the substance to the Priority Substances List (PSL) for further assessment, or recommending that the substance be added to the List of Toxic Substances in Schedule 1 and, where applicable, the implementation of virtual elimination. A screening assessment involves an analysis of a substance using conservative assumptions to determine whether the substance is “toxic” or capable of becoming “toxic” as defined in CEPA 1999. This environmental screening assessment examines various supporting information and develops conclusions based on a weight of evidence approach as required under Section 76.1 of CEPA 1999. The screening assessment does not present an exhaustive review of all available data; rather, it presents the most critical studies and lines of evidence supporting the conclusions. One line of evidence includes consideration of risk quotients to identify potential for ecological effects. However, other concerns that affect current or potential risk, such as persistence, bioaccumulation, chemical transformation and trends in ambient concentrations, are also examined in this report. -

Synthetic Studies of Diphenyl Ether and Anthraquinone Natural Products Ganeshkumar Lakshminarayan Iowa State University
Iowa State University Capstones, Theses and Graduate Theses and Dissertations Dissertations 2009 Synthetic studies of diphenyl ether and anthraquinone natural products Ganeshkumar Lakshminarayan Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/etd Part of the Chemistry Commons Recommended Citation Lakshminarayan, Ganeshkumar, "Synthetic studies of diphenyl ether and anthraquinone natural products" (2009). Graduate Theses and Dissertations. 11163. https://lib.dr.iastate.edu/etd/11163 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Graduate Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. Synthetic studies of diphenyl ether and anthraquinone natural products by Ganeshkumar Lakshminarayan A dissertation submitted to the graduate faculty in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY Major: Organic Chemistry Program of Study Committee: George A. Kraus, Major Professor Richard C. Larock John G. Verkade Klaus Schmidt-Rohr Suzanne Hendrich Iowa State University Ames, Iowa 2010 ii TABLE OF CONTENTS GENERAL INTRODUCTION 1 CHAPTER 1: Synthesis of littorachalcone and related diphenyl ethers Introduction 3 Results and discussion 5 Experimental section 16 References 24 CHAPTER 2 : An approach to the synthesis of