32 Electrostatics.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Electromagnetic Hypersensitivity
Electromagnetic Hypersensitivity Proceedings International Workshop on EMF Hypersensitivity Prague, Czech Republic October 25-27, 2004 Editors Kjell Hansson Mild Mike Repacholi Emilie van Deventer Paolo Ravazzani WHO Library Cataloguing-in-Publication Data: International Workshop on Electromagnetic Field Hypersensitivity (2004 : Prague, Czech Republic) Electromagnetic Hypersensitivity : proceedings, International Workshop on Electromagnetic Field Hypersensitivity, Prague, Czech Republic, October 25-27, 2004 / editors, Kjell Hansson Mild, Mike Repacholi, Emilie van Deventer, and Paolo Ravazzani. 1.Electromagnetic fields - adverse effects. 2.Hypersensitivity. 3.Environmental exposure. 4.Psychophysiologic disorders. I.Mild, Kjell Hansson. II.Repacholi, Michael H. III.Deventer, Emilie van. IV.Ravazzani, Paolo. V.World Health Organization. VI.Title. VII.Title: Proceedings, International Workshop on Electromagnetic Field Hypersensitivity, Prague, Czech Republic, October 25-27, 2004. ISBN 92 4 159412 8 (NLM classification: QT 34) ISBN 978 92 4 159412 7 © World Health Organization 2006 All rights reserved. Publications of the World Health Organization can be obtained from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel: +41 22 791 3264; fax: +41 22 791 4857; email: [email protected]). Requests for permission to reproduce or translate WHO publications – whether for sale or for noncommercial distribution – should be addressed to WHO Press, at the above address (fax: +41 22 791 4806; email: [email protected]). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. -

Handbook of Induction Heating Theoretical Background
This article was downloaded by: 10.3.98.104 On: 28 Sep 2021 Access details: subscription number Publisher: CRC Press Informa Ltd Registered in England and Wales Registered Number: 1072954 Registered office: 5 Howick Place, London SW1P 1WG, UK Handbook of Induction Heating Valery Rudnev, Don Loveless, Raymond L. Cook Theoretical Background Publication details https://www.routledgehandbooks.com/doi/10.1201/9781315117485-3 Valery Rudnev, Don Loveless, Raymond L. Cook Published online on: 11 Jul 2017 How to cite :- Valery Rudnev, Don Loveless, Raymond L. Cook. 11 Jul 2017, Theoretical Background from: Handbook of Induction Heating CRC Press Accessed on: 28 Sep 2021 https://www.routledgehandbooks.com/doi/10.1201/9781315117485-3 PLEASE SCROLL DOWN FOR DOCUMENT Full terms and conditions of use: https://www.routledgehandbooks.com/legal-notices/terms This Document PDF may be used for research, teaching and private study purposes. Any substantial or systematic reproductions, re-distribution, re-selling, loan or sub-licensing, systematic supply or distribution in any form to anyone is expressly forbidden. The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The publisher shall not be liable for an loss, actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material. 3 Theoretical Background Induction heating (IH) is a multiphysical phenomenon comprising a complex interac- tion of electromagnetic, heat transfer, metallurgical phenomena, and circuit analysis that are tightly interrelated and highly nonlinear because the physical properties of materi- als depend on magnetic field intensity, temperature, and microstructure. -

Upward Electrical Discharges from Thunderstorm Tops
UPWARD ELECTRICAL DISCHARGES FROM THUNDERSTORM TOPS BY WALTER A. LYONS, CCM, THOMAS E. NELSON, RUSSELL A. ARMSTRONG, VICTOR P. PASKO, AND MARK A. STANLEY Mesospheric lightning-related sprites and elves, not attached to their parent thunderstorm’s tops, are being joined by a family of upward electrical discharges, including blue jets, emerging directly from thunderstorm tops. or over 100 years, persistent eyewitness reports in (Wilson 1956). On the night of 6 July 1989, while the scientific literature have recounted a variety testing a low-light television camera (LLTV) for an Fof brief atmospheric electrical phenomena above upcoming rocket launch, the late Prof. John R. thunderstorms (Lyons et al. 2000). The startled ob- Winckler of the University of Minnesota made a most servers, not possessing a technical vocabulary with serendipitous observation. Replay of the video tape which to report their observations, used terms as var- revealed two frames showing brilliant columns of ied as “rocket lightning,” “cloud-to-stratosphere light extending far into the stratosphere above dis- lightning,” “upward lightning,” and even “cloud-to- tant thunderstorms (Franz et al. 1990). This single space lightning” (Fig. 1). Absent hard documenta- observation has energized specialists in scientific dis- tion, the atmospheric electricity community gave ciplines as diverse as space physics, radio science, at- little credence to such anecdotal reports, even one mospheric electricity, atmospheric acoustics, and originating with a Nobel Prize winner in physics -

Electrical Structure of the Stratosphere and Mesophere
1969 (6th) Vol. 1 Space, Technology, and The Space Congress® Proceedings Society Apr 1st, 8:00 AM Electrical Structure of the Stratosphere and Mesophere Willis L. Webb U.S. Army Electronics Command Follow this and additional works at: https://commons.erau.edu/space-congress-proceedings Scholarly Commons Citation Webb, Willis L., "Electrical Structure of the Stratosphere and Mesophere" (1969). The Space Congress® Proceedings. 1. https://commons.erau.edu/space-congress-proceedings/proceedings-1969-6th-v1/session-16/1 This Event is brought to you for free and open access by the Conferences at Scholarly Commons. It has been accepted for inclusion in The Space Congress® Proceedings by an authorized administrator of Scholarly Commons. For more information, please contact [email protected]. ELECTRICAL STRUCTURE OF THE STRATOSPHERE AND MESOSPHERE Will is L. V/ebb Atmospheric Sciences Laboratory U S Army Electronics Command White Sands Missile Range, New Mexico Synoptic rocket exploration of the strato exploration of the earth's upper atmosphere using spheric circulation has revealed the presence of small rocket vehicles was initiated to extend the hemispheric tidal circulations that are indicated region of meteorological study to higher alti to be in part characterized by systematic vertical tudes* . This meteorological rocket network (MRN) motions in low latitudes of the sunlit hemisphere. has expanded the atmospheric volume currently sub These vertical motions are powered by meridional ject to meteorological scrutiny from limitations oscillations in the stratospheric circulation pro of the order of 30-km peak altitude to a current duced by solar heating of the stratopause region synoptic data ceiling of the order of 80 km. -

Electrical Phenomena on the Moon and Mars
Proc. ESA Annual Meeting on Electrostatics 2010, Paper A1 Electrical Phenomena on the Moon and Mars Gregory T. Delory Space Sciences Laboratory University of California, Berkeley phone: (1) 510-643-1991 e-mail: [email protected] Abstract—The Moon and Mars represent intriguing and divergent case studies where nat- ural electrical processes may occur in environments beyond our more familiar terrestrial experience. The windy, Aeolian environment of Mars likely produces substantial electrical activity via the tribo-electrification of individual dust grains that occurs during atmospheric disturbances. While there may be some analogies between atmospheric electrical processes on the Earth and Mars, the highly rarefied, dry Martian atmosphere imposes unique conditions that govern the charging and discharge dynamics of particulates. In contrast to the wind- swept surface of Mars, the Moon is a small airless body whose surface is directly exposed to variable space plasmas and solar irradiation. Measurements during the Apollo missions, to- gether with more recent data from orbital spacecraft, indicate that there are active and dy- namic charging processes occurring on and near the lunar surface. One possible consequence of dynamic lunar electrical activity may be the levitation and perhaps large scale transport of lunar dust. For both the Moon and Mars we only have indirect evidence at best for the exis- tence of electrical activity of any real global consequence. This paper is a brief, semi-tutorial review that discusses the background and history behind these investigations, highlights key ongoing research, and describes future efforts that will help resolve the fundamental, out- standing questions that remain. -

Intensification of Electrohydrodynamic Flows Using Carbon Nano-Tubes
Journal of Physics: Conference Series PAPER • OPEN ACCESS Intensification of electrohydrodynamic flows using carbon nano-tubes To cite this article: S M Korobeynikov et al 2020 J. Phys.: Conf. Ser. 1675 012103 View the article online for updates and enhancements. This content was downloaded from IP address 170.106.202.226 on 25/09/2021 at 00:59 TPH-2020 IOP Publishing Journal of Physics: Conference Series 1675 (2020) 012103 doi:10.1088/1742-6596/1675/1/012103 Intensification of electrohydrodynamic flows using carbon nano-tubes S M Korobeynikov1,2, A V Ridel1,2, D I Karpov1,3, Ya G Prokopenko3 and A L Bychkov1 1 Lavrentyev institute of hydrodynamics of SB RAS, Lavrentyev av., 15, Novosibirsk, 630090 2 Novosibirsk State Technical University, Karl Marx av., 20, Novosibirsk, 630073 3 Novosibirsk State University, Pirogova str., 2, Novosibirsk, 630090 E-mail: [email protected] Abstract. Study of behaviour of floating up bubbles in the mixture of transformer oil with carbon nanotubes under the action of high electric field was carried out. The possibility of using carbon nanotubes to create hydrodynamic flows in a liquid dielectric at a very low concentration of nanotubes is shown. It is assumed that the mechanism of the formation of flows is the injection of charge from the surface of nanotubes. 1. Introduction The study of partial discharges (PDs) in liquid dielectrics has quite a long history. Despite this it remains a very important field of physics of electrical phenomena in dielectrics. There are two basic factors determining practical significance of these studies. All possible PDs events in dielectric liquids are divided into two primary types. -

Ilmimfthe Original
. DOCUNEIT RESURE ED. 123 059 SE 020 447 AUTHOR' Baker, Thomas S.; lei her, John P. ?r11.2 Equinox. A model for -the Natural.Science.Edacation Curriculum for the Ninth Through'Twelfth Grades, in the Delaware Schools. INFZITUTION Deliiiii State Dept. of Public Instimctiod, Dover% Del' IIadSystem,' Dovei, Deli SPONS AGENCY...,.., National Science Foundation, Washington,'D,C. PUB DATE Jan 75 : NSP-GW-6703 .;-,- APVT ." - . NoW : 16p.: For related docuaeats, see SE 019 380 And SE 020 404-406; lest Copy Avitlable; Colored Paper AVAILABLE P4OS Er. John P..1eiher, State Supervisor -ef Science and Envirolftental Educatitin, Department of Public Instruction, John G. Townsend Building, Dover, ..1Ddelavare 1991 (Prep while supply lasts). .. !DRS PRICE SP-58.83 EC-$2.4 plus Postage DESCRIPTORS Biological Sciences; *Curriculum Guides; *Natural 'ScienCes; Physical Sciences; :Science 'Education; *Secondary Education: *Secondary School Science; State Curriculum Guides; State Programs mixeliTimm *Delaware; Del Sod System: National Science Poundation; RSP ABSTRACT This publication represents a soael for the Natural Science Education Curriculum for grades nine through twelve in Delawares ichools. This guide it .meant to serveas a minimal .standard for natural'science education, but ,at the sane time strives -- for maxi"um output of the natural science program. The guide is based on the processes of science education as well as the concepts and attitudes of the biological, physical', and earth sciences. Four basic goals have been identified and a set of terminal objectives has been ev. established for each goal. These goals and objectives provide the framework for the development of district, local, building, or classroot-programs. -
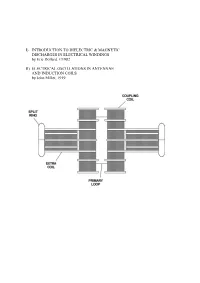
I) Introduction to Dielectric & Magnetic Discharges In
I) INTRODUCTION TO DIELECTRIC & MAGNETIC DISCHARGES IN ELECTRICAL WINDINGS by Eric Dollard, ©1982 II) ELECTRICAL OSCILLATIONS IN ANTENNAE AND INDUCTION COILS by John Miller, 1919 PART I INTRODUCTION TO DIELECTRIC & MAGNETIC DISCHARGES IN ELECTRICAL WINDINGS by Eric Dollard, ©1982 1. CAPACITANCE 2. CAPACITANCE INADEQUATELY EXPLAINED 3. LINES OF FORCE AS REPRESENTATION OF DIELECTRICITY 4. THE LAWS OF LINES OF FORCE 5. FARADAY'S LINES OF FORCE THEORY 6. PHYSICAL CHARACTERISTICS OF LINES OF FORCE 7. MASS ASSOCIATED WITH LINES OF FORCE IN MOTION 8. INDUCTANCE AS AN ANALOGY TO CAPACITANCE 9. MECHANISM OF STORING ENERGY MAGNETICALLY 10. THE LIMITS OF ZERO AND INFINITY 11. INSTANT ENERGY RELEASE AS INFINITY 12. ANOTHER FORM OF ENERGY APPEARS 13. ENERGY STORAGE SPATIALLY DIFFERENT THAN MAGNETIC ENERGY STORAGE 14. VOTAGE IS TO DIELECTRICITY AS CURRENT IS TO MAGNETISM 15. AGAIN THE LIMITS OF ZERO AND INFINITY 16. INSTANT ENERGY RELEASE AS INFINITY 17. ENERGY RETURNS TO MAGNETIC FORM 18. CHARACTERISTIC IMPEDANCE AS A REPRESENTATION OF PULSATION OF ENERGY 19. ENERGY INTO MATTER 20. MISCONCEPTION OF PRESENT THEORY OF CAPACITANCE 21. FREE SPACE INDUCTANCE IS INFINITE 22. WORK OF TESLA, STEINMETZ, AND FARADAY 23. QUESTION AS TO THE VELOCITY OF DIELECTRIC FLUX APPENDIX I 0) Table of Units, Symbols & Dimensions 1) Table of Magnetic & Dielectric Relations 2) Table of Magnetic, Dielectric & Electronic Relations PART II ELECTRICAL OSCILLATIONS IN ANTENNAE & INDUCTION COILS J.M. Miller Proceedings, Institute of Radio Engineers. 1919 1) CAPACITANCE The phenomena of capacitance is a type of electrical energy storage in the form of a field in an enclosed space. This space is typically bounded by two parallel metallic plates or two metallic foils on an interviening insulator or dielectric. -

Electrical Phenomena
ELECTRICAL PHENOMENA OBJECTIVES • To describe qualitatively the phenomena of electrification, electrostatic attraction and repulsion and to introduce the concepts of electric charge, insulators and conductors. • To justify Coulomb's law and use it to calculate the force exerted by one or two charged particles on another. • To justify field intensity and calculate its value in the case of one or two charged particles. • To describe the electric field using force lines. • To justify the concepts of potential electric energy and potential, as well as using them to calculate the work done when moving a particle in the electric field. • To observe the relation between field intensity and potential. • To carry out some predictions on the movement of charges in uniform fields. 1.1 What we already know about electricity At some time all of us have done the experiment of "electrifying" our pen by rubbing it on the sleeve of our jersey so that it attracts little pieces of paper. In previous years we have learned that the atoms of a material possess particles with the property we call an electric charge. We know that there are positive charges in the nucleus called protons, and negative charges outside the nucleus called electrons. Could you explain what type of charges have moved when we charge our pen? © Proyecto Newton. MEC. José L. San Emeterio The natural unit of electric charge should be the electron. But as it is too small for practical purposes we have adopted another unit called the coulomb, equivalent to the charge of some 6 trillion electrons. We will give the exact definition of this unit in the topic on electric current, in the section on electrolysis. -

Indirect Effects of Lightning Discharges
SERBIAN JOURNAL OF ELECTRICAL ENGINEERING Vol. 8, No. 3, November 2011, 245-262 UDK: 551.594.2:537.8; 621.316.932 DOI: 10.2298/SJEE1103245P Indirect Effects of Lightning Discharges Gururaj S. Punekar1, Chandrasekaran Kandasamy2 Abstract: The occurrence of lightning strokes due to indirect effect of lightning discharges, has assumed a lot of importance in the recent times. This is due to the sensitive, vital electronic equipment which are highly vulnerable to such indirect effects. In this article, attempts are made to bring out the salient features and related parameters of lightning discharges (with specific reference to indirect effects). Glimpses of the experimental research efforts to understand the phenomenon are described based on the published scientific work, along with some of the typical simulation results of the authors. These simulation results (computed electromagnetic fields) are validated by some of the important results described in the literature. This being a review article, the vital electrical and electronic systems/components which have been researched with reference to indirect effects have been enumerated, and the present understandings have been discussed. Keywords: Electric stress, Electromagnetic fields, Indirect effect, Lightning, Over voltages. 1 Introduction Lightning is a natural electrical phenomena being the most spectacular to every common man. Of all lightning discharges only around 25% of the lightning bolt reaches the ground. Lightning being an intense power source (although of short duration), has the potential to cause significant damage to life and property. Attempts to understand the phenomena (being most spectacular in nature but destructive), has been a great challenge and forms one of the well researched area. -

Continuous Natural Vector Theory of Electromagnetism H
UET2 Continuous Natural Vector Theory of Electromagnetism H. J. Spencer * II ABSTRACT A new algebraic representation is used to immediately recover all the major results of classical electromagnetism. This new representation (‘Natural Vectors’) is based on Hamilton’s quaternions and completes the original attempt by Maxwell to use this powerful, non-commutative algebra in the final presentation of his theory in his Treatise. The foundational hypothesis here is that the principal electromagnetic variables are best represented by Natural Vectors, rather than the conventional 3D vectors defined by ‘real numbers’. The present results avoid all use of the field concept and validate the retarded scalar and vector potentials approach first introduced by L. V. Lorenz, who combined Gauss’s 1845 suggestion of the finite speed of interaction with Newton’s action-at-a-distance model of physics into a charge-potential model of electromagnetism in 1867. This new approach demonstrates the primacy and physical significance of the ‘Lorenz gauge’. Not withstanding Maxwell’s aether theory, the present results are based on the continuous charge-density substance model of electricity that is used today to develop Maxwell’s Equations for classical electromagnetism. The present analysis also demonstrates that Helmholtz’s ‘fluid’ model of electricity is one of the few that can result in an electromagnetic ‘explanation’ for the phenomenon of light. Unlike algebraic Minkowski 4-vectors, the more powerful 4-dimensional covariant Natural Vectors used here generate all the differential equations normally found in classical electromagnetism in an immediate and direct algebraic manner. This new theory focuses on the remote interaction between charges, which then appears both as variations in the charge-density and the potentials “traveling at light-speed across space”. -

SQA Advanced Higher Physics Unit 2: Electrical Phenomena
SCHOLAR Study Guide SQA Advanced Higher Physics Unit 2: Electrical Phenomena Andrew Tookey Heriot-Watt University Campbell White Tynecastle High School Heriot-Watt University Edinburgh EH14 4AS, United Kingdom. First published 2001 by Heriot-Watt University. This edition published in 2013 by Heriot-Watt University SCHOLAR. Copyright © 2013 Heriot-Watt University. Members of the SCHOLAR Forum may reproduce this publication in whole or in part for educational purposes within their establishment providing that no profit accrues at any stage, Any other use of the materials is governed by the general copyright statement that follows. All rights reserved. No part of this publication may be reproduced, stored in a retrieval system or transmitted in any form or by any means, without written permission from the publisher. Heriot-Watt University accepts no responsibility or liability whatsoever with regard to the information contained in this study guide. Distributed by Heriot-Watt University. SCHOLAR Study Guide Unit 2: SQA Advanced Higher Physics 1. SQA Advanced Higher Physics ISBN 978-1-906686-07-9 Printed and bound in Great Britain by Graphic and Printing Services, Heriot-Watt University, Edinburgh. Acknowledgements Thanks are due to the members of Heriot-Watt University's SCHOLAR team who planned and created these materials, and to the many colleagues who reviewed the content. We would like to acknowledge the assistance of the education authorities, colleges, teachers and students who contributed to the SCHOLAR programme and who evaluated these materials. Grateful acknowledgement is made for permission to use the following material in the SCHOLAR programme: The Scottish Qualifications Authority for permission to use Past Papers assessments.