Factors Affecting Early Life Patterns of the European Flounder Platichthys Flesus in a Nursery Habitat
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Redalyc.A Review of the Flatfish Fisheries of the South Atlantic Ocean
Revista de Biología Marina y Oceanografía ISSN: 0717-3326 [email protected] Universidad de Valparaíso Chile Díaz de Astarloa, Juan M. A review of the flatfish fisheries of the south Atlantic Ocean Revista de Biología Marina y Oceanografía, vol. 37, núm. 2, diciembre, 2002, pp. 113-125 Universidad de Valparaíso Viña del Mar, Chile Available in: http://www.redalyc.org/articulo.oa?id=47937201 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Revista de Biología Marina y Oceanografía 37 (2): 113 - 125, diciembre de 2002 A review of the flatfish fisheries of the south Atlantic Ocean Una revisión de las pesquerías de lenguados del Océano Atlántico sur Juan M. Díaz de Astarloa1 2 1CONICET, Departamento de Ciencias Marinas, Facultad de Ciencias Exactas y Naturales, Universidad Nacional de Mar del Plata, Funes 3350, 7600 Mar del Plata, Argentina. [email protected] 2 Current address: Laboratory of Marine Stock-enhancement Biology, Division of Applied Biosciences, Graduate School of Agriculture, Kyoto University, kitashirakawa-oiwakecho, sakyo-ku, Kyoto, 606-8502 Japan. [email protected] Resumen.- Se describen las pesquerías de lenguados del Abstract.- The flatfish fisheries of the South Atlantic Atlántico sur sobre la base de series de valores temporales de Ocean are described from time series of landings between desembarcos pesqueros entre los años 1950 y 1998, e 1950 and 1998 and available information on species life información disponible sobre características biológicas, flotas, history, fleets and gear characteristics, and economical artes de pesca e importancia económica de las especies importance of commercial species. -
Schedule Onlinepdf
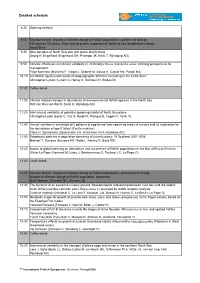
Detailed schedule 8:30 Opening session 9:00 Keynote lecture: Impacts of climate change on flatfish populations - patterns of change 100 days to 100 years: Short and long-term responses of flatfish to sea temperature change David Sims 9:30 Nine decades of North Sea sole and plaice distributions Georg H. Engelhard (Engelhard GH, Pinnegar JK, Kell LT, Rijnsdorp AD) 9:50 Climatic effects on recruitment variability in Platichthys flesus and Solea solea: defining perspectives for management. Filipe Martinho (Martinho F, Viegas I, Dolbeth M, Sousa H, Cabral HN, Pardal MA) 10:10 Are flatfish species with southern biogeographic affinities increasing in the Celtic Sea? Christopher Lynam (Lynam C, Harlay X, Gerritsen H, Stokes D) 10:30 Coffee break 11:00 Climate related changes in abundance of non-commercial flatfish species in the North Sea Ralf van Hal (van Hal R, Smits K, Rijnsdorp AD) 11:20 Inter-annual variability of potential spawning habitat of North Sea plaice Christophe Loots (Loots C, Vaz S, Koubii P, Planque B, Coppin F, Verin Y) 11:40 Annual variation in simulated drift patterns of egg/larvae from spawning areas to nursery and its implication for the abundance of age-0 turbot (Psetta maxima) Claus R. Sparrevohn (Sparrevohn CR, Hinrichsen H-H, Rijnsdorp AD) 12:00 Broadscale patterns in population dynamics of juvenile plaice: W Scotland 2001-2008 Michael T. Burrows (Burrows MT, Robb L, Harvey R, Batty RS) 12:20 Impact of global warming on abundance and occurrence of flatfish populations in the Bay of Biscay (France) Olivier Le Pape (Hermant -

Aspects of the Life History of Hornyhead Turbot, Pleuronichthys Verticalis, Off Southern California
Aspects of the Life History of Hornyhead Turbot, Pleuronichthys verticalis, off Southern California he hornyhead turbot T(Pleuronichthys verticalis) is a common resident flatfish on the mainland shelf from Magdalena Bay, Baja Califor- nia, Mexico to Point Reyes, California (Miller and Lea 1972). They are randomly distributed over the bottom at a density of about one fish per 130 m2 and lie partially buried in the sediment (Luckinbill 1969). Hornyhead turbot feed primarily on sedentary, tube-dwelling polychaetes (Luckinbill 1969, Allen 1982, Cross et al. 1985). They pull the tubes from the sediment, Histological section of a fish ovary. extract the polychaete, and then eject the tube (Luckinbill 1969). Hornyhead turbot are Orange County, p,p’-DDE Despite the importance of batch spawners and may averaged 362 μg/kg wet the hornyhead turbot in local spawn year round (Goldberg weight in hornyhead turbot monitoring programs, its life 1982). Their planktonic eggs liver and 5 μg/kg dry weight in history has received little are 1.00-1.16 mm diameter the sediments (CSDOC 1992). attention. The long-term goal (Sumida et al. 1979). Their In the same year in Santa of our work is to determine larvae occur in the nearshore Monica Bay, p,p’-DDE aver- how a relatively low trophic plankton throughout the year aged 7.8 mg/kg wet weight in level fish like the hornyhead (Gruber et al. 1982, Barnett et liver and 81 μg/kg dry weight turbot accumulates tissue al. 1984, Moser et al. 1993). in the sediments (City of Los levels of chlorinated hydrocar- Several agencies in South- Angeles 1992). -

Early Stages of Fishes in the Western North Atlantic Ocean Volume
ISBN 0-9689167-4-x Early Stages of Fishes in the Western North Atlantic Ocean (Davis Strait, Southern Greenland and Flemish Cap to Cape Hatteras) Volume One Acipenseriformes through Syngnathiformes Michael P. Fahay ii Early Stages of Fishes in the Western North Atlantic Ocean iii Dedication This monograph is dedicated to those highly skilled larval fish illustrators whose talents and efforts have greatly facilitated the study of fish ontogeny. The works of many of those fine illustrators grace these pages. iv Early Stages of Fishes in the Western North Atlantic Ocean v Preface The contents of this monograph are a revision and update of an earlier atlas describing the eggs and larvae of western Atlantic marine fishes occurring between the Scotian Shelf and Cape Hatteras, North Carolina (Fahay, 1983). The three-fold increase in the total num- ber of species covered in the current compilation is the result of both a larger study area and a recent increase in published ontogenetic studies of fishes by many authors and students of the morphology of early stages of marine fishes. It is a tribute to the efforts of those authors that the ontogeny of greater than 70% of species known from the western North Atlantic Ocean is now well described. Michael Fahay 241 Sabino Road West Bath, Maine 04530 U.S.A. vi Acknowledgements I greatly appreciate the help provided by a number of very knowledgeable friends and colleagues dur- ing the preparation of this monograph. Jon Hare undertook a painstakingly critical review of the entire monograph, corrected omissions, inconsistencies, and errors of fact, and made suggestions which markedly improved its organization and presentation. -

NHBSS 034 1G Wongratana R
NAT. NAT. HIST. BULL. SIAM So c. 34 (1) :65 ・70 ,1986 RECORD OF AMBICOLORATION IN CYNOGLOSSUS (PISCES : CYNOGLOSSIDAE) FROM THAILAND Thosaporn Thosaporn Wongratana * ABSTRACT An almost ambico10rate “Four- Ii ned tongu e- sole" ,Cynoglossus bilinealus (La cepede) , is is reported from Thailand. It is presumab1y the first record for the genus. Except for most of of the head on the blind side and its.corresponding finrays ,which are pale as in normal specimens ,the body and fins are pigmented. The norma Jl y cycloid scales on the blind side in in the pigmented area are who Jl y replaced by ctenoid scales ,but those on the unpigmented part part on the head are cyc10id. The latera1line sca1es of the pigmented area on the blind side are are cycloid. The pelvic fins are entirely separated from the anal fin by the absence of membrane. membrane. No other major externa1 anomaly is found. PREVIOUS ACCOUNTS Abnormalities Abnormalities in coloration 釘 e more common among members of the order Pleuronectiformes Pleuronectiformes than in any other group of fishes. Other anomalies occasionally in those those fishes are a hooked dorsal fin , incomplete eye migration and side reversa l. Abnormal pigmentation in flatfish is divided into three main types: ambicoloration , albinism ,and xanthochromism (DEVEEN , 1969; COLMAN ,1972 勾). P 町 “tia 剖10 町Ir incomplete ambic ∞010 町r羽 ion is mor 問ec ∞ommon than trunk pigmentation , nearly complete amb 凶ic ∞0- lor 問at “ion and complete ambic ∞010 町ra 創ti 拘on (υJONES & MENON , 1950). NORMAN'S (1 934) previous previous explanation of this phenomenon ,later accepted by many authors ,was that “..訓 nbicoloration merely represents variation in the direction of the original bilateral symmetrical symmetrical condition of the ancester of flatfishes." It is also regularly observed that that wholly ambicolorate fishes normally display a higher degree of symmetry than normal normal specimens in pigmentation ,scales , paired fins and final position of the eyes (NORMAN , 1934; COLMAN , 1972). -

The Landing Obligation and Its Implications on the Control of Fisheries
DIRECTORATE-GENERAL FOR INTERNAL POLICIES POLICY DEPARTMENT B: STRUCTURAL AND COHESION POLICIES FISHERIES THE LANDING OBLIGATION AND ITS IMPLICATIONS ON THE CONTROL OF FISHERIES STUDY This document was requested by the European Parliament's Committee on Fisheries. AUTHORS Ocean Governance Consulting: Christopher Hedley Centre for Environment, Fisheries and Aquaculture Science: Tom Catchpole, Ana Ribeiro Santos RESPONSIBLE ADMINISTRATOR Marcus Breuer Policy Department B: Structural and Cohesion Policies European Parliament B-1047 Brussels E-mail: [email protected] EDITORIAL ASSISTANCE Adrienn Borka Lyna Pärt LINGUISTIC VERSIONS Original: EN ABOUT THE PUBLISHER To contact the Policy Department or to subscribe to its monthly newsletter please write to: [email protected] Manuscript completed in September 2015. © European Union, 2015. Print ISBN 978-92-823-7938-7 doi:10.2861/694624 QA-02-15-709-EN-C PDF ISBN 978-92-823-7939-4 doi:10.2861/303902 QA-02-15-709-EN-N This document is available on the Internet at: http://www.europarl.europa.eu/studies DISCLAIMER The opinions expressed in this document are the sole responsibility of the author and do not necessarily represent the official position of the European Parliament. Reproduction and translation for non-commercial purposes are authorized, provided the source is acknowledged and the publisher is given prior notice and sent a copy. DIRECTORATE-GENERAL FOR INTERNAL POLICIES POLICY DEPARTMENT B: STRUCTURAL AND COHESION POLICIES FISHERIES THE LANDING OBLIGATION AND ITS IMPLICATIONS ON THE CONTROL OF FISHERIES STUDY Abstract This study reviews the impacts of the new Common Fisheries Policy (CFP) rules requiring catches in regulated fisheries to be landed and counted against quotas of each Member State ("the landing obligation and requiring that catch of species subject to the landing obligation below a minimum conservation reference size be restricted to purposes other than direct human consumption. -

The Influence of Hediste Diversicolor (O.F
Rostock. Meeresbiolog. Beitr. (1993)1 Andreas Bick; Günter Arlt The influence of Hediste diversicolor (O.F. MÜLLER, 1776) on the macro- and meiozoobenthos of a shallow water area of Mecklenburg Bay (Western Baltic Sea) Introduction The purpose of many ecological studies is to identify interactions between faunistic ecosystem components by means of laboratory and field experiments. It has often been shown that abiotic and biotic factors such as competition, disturbance and predation influence the composition and dynamics of macrobenthos communities (REISE, 1977; COMMITO and AMBROSE, 1985; AM- BROSE, 1986; BEUKEMA, 1987; KIKUCHI, 1987; REDMOND and SCOTT, 1989; COMMITO and BANCAVAGE, 1989; MATILA and BONSDORFF, 1989; HILL et al., 1990). Experiments have also been performed to detect interactions between macrofauna and meiofauna (BELL & Coull, 1978; REISE, 1979; GEE et al., 1985; ALONGI and TENORE, 1985). The interactions between Nereidae and infauna that are common in shallow water and are fairly easily handled have been a major topic of study (COMMITO, 1982; REISE, 1979b; COMMITO and SCHRADER, 1985; OLAFSON and PERSSON 1986; RÖNN et al., 1988). The purpose of our studies was to investigate the influence of the omnivorous H. diversicolor on the infauna of a shallow water region in the southern Baltic Sea. H. diversicolor achieves abun- dances of between 5,000 and 15,000 ind m"2 (individual dominances up to 15 %) in the investi- gation area and is thus a major component of the macrofauna. It therefore seemed likely that its carnivorous feeding habits can affect community structure. To detect direct influences on both the macrozoobenthos and meiozoobenthos and to reduce box effects, we performed short term experiments. -

The State of Mediterranean and Black Sea Fisheries 2018
Food and Agriculture General Fisheries Commission for the Mediterranean Organization of the Commission générale des pêches United Nations pour la Méditerranée ISSN 2413-6905 THE STATE OF MEDITERRANEAN AND BLACK SEA FISHERIES 2018 Reference: FAO. 2018. The State of Mediterranean and Black Sea Fisheries General Fisheries Commission for the Mediterranean. Rome, Italy. pp. 164. THE STATE OF MEDITERRANEAN AND BLACK SEA FISHERIES 2018 FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS Rome, 2018 Required citation: FAO. 2018. The State of Mediterranean and Black Sea Fisheries. General Fisheries Commission for the Mediterranean. Rome. 172 pp. The designations employed and the presentation of material in this information product do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations (FAO) concerning the legal or development status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. The mention of specifc companies or products of manufacturers, whether or not these have been patented, does not imply that these have been endorsed or recommended by FAO in preference to others of a similar nature that are not mentioned. The views expressed in this information product are those of the author(s) and do not necessarily refect the views or policies of FAO. ISBN 978-92-5-131152-3 © FAO, 2018 Some rights reserved. This work is made available under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 IGO licence (CC BY-NC-SA 3.0 IGO; https://creativecommons.org/licenses/by-nc-sa/3.0/igo/legalcode/legalcode). -

Wholesale Market Profiles for Alaska Groundfish and Crab Fisheries
JANUARY 2020 Wholesale Market Profiles for Alaska Groundfish and FisheriesCrab Wholesale Market Profiles for Alaska Groundfish and Crab Fisheries JANUARY 2020 JANUARY Prepared by: McDowell Group Authors and Contributions: From NOAA-NMFS’ Alaska Fisheries Science Center: Ben Fissel (PI, project oversight, project design, and editor), Brian Garber-Yonts (editor). From McDowell Group, Inc.: Jim Calvin (project oversight and editor), Dan Lesh (lead author/ analyst), Garrett Evridge (author/analyst) , Joe Jacobson (author/analyst), Paul Strickler (author/analyst). From Pacific States Marine Fisheries Commission: Bob Ryznar (project oversight and sub-contractor management), Jean Lee (data compilation and analysis) This report was produced and funded by the NOAA-NMFS’ Alaska Fisheries Science Center. Funding was awarded through a competitive contract to the Pacific States Marine Fisheries Commission and McDowell Group, Inc. The analysis was conducted during the winter of 2018 and spring of 2019, based primarily on 2017 harvest and market data. A final review by staff from NOAA-NMFS’ Alaska Fisheries Science Center was completed in June 2019 and the document was finalized in March 2016. Data throughout the report was compiled in November 2018. Revisions to source data after this time may not be reflect in this report. Typically, revisions to economic fisheries data are not substantial and data presented here accurately reflects the trends in the analyzed markets. For data sourced from NMFS and AKFIN the reader should refer to the Economic Status Report of the Groundfish Fisheries Off Alaska, 2017 (https://www.fisheries.noaa.gov/resource/data/2017-economic-status-groundfish-fisheries-alaska) and Economic Status Report of the BSAI King and Tanner Crab Fisheries Off Alaska, 2018 (https://www.fisheries.noaa. -

A Review of Soles of the Genus Aseraggodes from the South Pacific, with Descriptions of Seven New Species and a Diagnosis of Synclidopus
Memoirs of Museum Victoria 62(2): 191–212 (2005) ISSN 1447-2546 (Print) 1447-2554 (On-line) http://www.museum.vic.gov.au/memoirs/index.asp A review of soles of the genus Aseraggodes from the South Pacific, with descriptions of seven new species and a diagnosis of Synclidopus. JOHN E. RANDALL Bishop Museum, 1525 Bernice St. Honolulu, Hawai’i 96817–2704, USA ([email protected]) Abstract Randall, J.E. 2005. A review of soles of the genus Aseraggodes from the South Pacific, with descriptions of seven new species and a diagnosis of Synclidopus. Memoirs of Museum Victoria 62(2): 191–212 The soleid genus Parachirus Matsubara and Ochiai is referred to the synonymy of Aseraggodes Kaup. Aseraggodes persimilis (Günther) and A. ocellatus Weed are reclassified in the genus Pardachirus Günther. Synclidopus macleayanus (Ramsay) from Queensland and NSW is redescribed. A diagnosis is given for Aseraggodes, and a key and species accounts provided for the following 12 species of the genus from islands of Oceania in the South Pacific and the east coast of Australia: A. auroculus, sp. nov. from the Society Islands; A. bahamondei from Easter Island and Lord Howe Island; A. cyclurus, sp. nov. from the Society Islands; A. lateralis, sp. nov. from the Marquesas Islands; A. lenisquamis, sp. nov. from NSW; A. magnoculus sp. nov. from New Caledonia; A. melanostictus (Peters) from 73 m off Bougainville and a first record for the Great Barrier Reef from 115 m; A. nigrocirratus, sp. nov. from NSW; A. normani Chabanaud from southern Queensland and NSW; A. pelvicus, sp. nov. -

(Paralichthys Lethostigma) in the Galveston Bay Estuary, TX
DISTRIBUTION, CONDITION, AND GROWTH OF NEWLY SETTLED SOUTHERN FLOUNDER (Paralichthys lethostigma) IN THE GALVESTON BAY ESTUARY, TX A Thesis by LINDSAY ANN GLASS Submitted to the Office of Graduate Studies of Texas A&M University in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE May 2006 Major Subject: Wildlife and Fisheries Sciences DISTRIBUTION, CONDITION, AND GROWTH OF NEWLY SETTLED SOUTHERN FLOUNDER (Paralichthys lethostigma) IN THE GALVESTON BAY ESTUARY, TX A Thesis by LINDSAY ANN GLASS Submitted to the Office of Graduate Studies of Texas A&M University in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE Approved by: Chair of Committee, Jay R. Rooker Committee Members, William H. Neill Antonietta Quigg Head of Department, Delbert M.Gatlin III May 2006 Major Subject: Wildlife and Fisheries Sciences iii ABSTRACT Distribution, Condition, and Growth of Newly Settled Southern Flounder (Paralichthys lethostigma) in the Galveston Bay Estuary, TX. (May 2006) Lindsay Ann Glass, B.S., Texas A&M University-Galveston Chair of Advisory Committee: Dr. Jay R. Rooker Several flatfish species including southern flounder (Paralichthys lethostigma) recruit to estuaries during early life. Therefore, the evaluation of estuarine sites and habitats that serve as nurseries is critical to conservation and management efforts. I used biochemical condition and growth measurements in conjunction with catch-density data to evaluate settlement sites used by southern flounder in the Galveston Bay Estuary (GBE). In 2005, beam-trawl collections were made in three major sections of the GBE (East Bay, West Bay, Galveston Bay), and three sites were sampled in each bay. -

Updated Checklist of Marine Fishes (Chordata: Craniata) from Portugal and the Proposed Extension of the Portuguese Continental Shelf
European Journal of Taxonomy 73: 1-73 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2014.73 www.europeanjournaloftaxonomy.eu 2014 · Carneiro M. et al. This work is licensed under a Creative Commons Attribution 3.0 License. Monograph urn:lsid:zoobank.org:pub:9A5F217D-8E7B-448A-9CAB-2CCC9CC6F857 Updated checklist of marine fishes (Chordata: Craniata) from Portugal and the proposed extension of the Portuguese continental shelf Miguel CARNEIRO1,5, Rogélia MARTINS2,6, Monica LANDI*,3,7 & Filipe O. COSTA4,8 1,2 DIV-RP (Modelling and Management Fishery Resources Division), Instituto Português do Mar e da Atmosfera, Av. Brasilia 1449-006 Lisboa, Portugal. E-mail: [email protected], [email protected] 3,4 CBMA (Centre of Molecular and Environmental Biology), Department of Biology, University of Minho, Campus de Gualtar, 4710-057 Braga, Portugal. E-mail: [email protected], [email protected] * corresponding author: [email protected] 5 urn:lsid:zoobank.org:author:90A98A50-327E-4648-9DCE-75709C7A2472 6 urn:lsid:zoobank.org:author:1EB6DE00-9E91-407C-B7C4-34F31F29FD88 7 urn:lsid:zoobank.org:author:6D3AC760-77F2-4CFA-B5C7-665CB07F4CEB 8 urn:lsid:zoobank.org:author:48E53CF3-71C8-403C-BECD-10B20B3C15B4 Abstract. The study of the Portuguese marine ichthyofauna has a long historical tradition, rooted back in the 18th Century. Here we present an annotated checklist of the marine fishes from Portuguese waters, including the area encompassed by the proposed extension of the Portuguese continental shelf and the Economic Exclusive Zone (EEZ). The list is based on historical literature records and taxon occurrence data obtained from natural history collections, together with new revisions and occurrences.