Kites, Eagles, Hawks and Alli
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Eastern Marsh Harrier Chu-Hi (Jpn) Circus Spilonotus Morphology and Classification Still Undiscovered Nesting Grounds in Hokkaido in Particular
Bird Research News Vol.7 No.5 2010.5.20. Eastern Marsh Harrier Chu-hi (Jpn) Circus spilonotus Morphology and classification still undiscovered nesting grounds in Hokkaido in particular. The total population of the species wintering in Japan, on the other hand, has not been counted except for the roosting number of some Classification: Accipitriformes Accipitridae areas, such as Watarase Marsh, Tochigi Pref., central Japan. Total length: ♂ 480mm ♀ 580mm Wing length: 380-430mm Wingspan: 1132-1372mm Nest: Tail length: 215-262mm Culmen length: 28-31mm They build a nest in wet reed beds or the dry tall grassland of Japa- Tarsus length: 85-91mm Weight: 498-844g nese pampas grass (Miscanthus sinensis), etc., piling up dry grass on the ground (Nishide 1979, Tada 2007, Naya et. al. 2007, Chiba Measurements after Enomoto (1941). 2008). The nest size is about 110-130cm by 80-90cm (Chiba 2008, Naya et al. 2007). Appearance: The plumage coloration of East- Egg: ern Marsh Harriers is basically They lay an egg at 3.3 day intervals on average (Nishide 1979). brownish, but varies considera- The clutch size is 4-7 eggs (Chiba 2008, Nishide 1979). The egg bly (Morioka et al. 1995). There size is 48.0mm by 38.0mm on average (n = 5) (Chiba 2008). The are types such as totally dark egg color is grayish white (Chiba 2008). brown, off-white from the head to the leading edge of a wing, Incubation and nestling periods: and pale brown with a vertical- Females mostly incubate eggs. The incubation period is about 28- striped underpart, bluish gray 34 days (Chiba 2008). -

A Multi-Gene Phylogeny of Aquiline Eagles (Aves: Accipitriformes) Reveals Extensive Paraphyly at the Genus Level
Available online at www.sciencedirect.com MOLECULAR SCIENCE•NCE /W\/Q^DIRI DIRECT® PHYLOGENETICS AND EVOLUTION ELSEVIER Molecular Phylogenetics and Evolution 35 (2005) 147-164 www.elsevier.com/locate/ympev A multi-gene phylogeny of aquiline eagles (Aves: Accipitriformes) reveals extensive paraphyly at the genus level Andreas J. Helbig'^*, Annett Kocum'^, Ingrid Seibold^, Michael J. Braun^ '^ Institute of Zoology, University of Greifswald, Vogelwarte Hiddensee, D-18565 Kloster, Germany Department of Zoology, National Museum of Natural History, Smithsonian Institution, 4210 Silver Hill Rd., Suitland, MD 20746, USA Received 19 March 2004; revised 21 September 2004 Available online 24 December 2004 Abstract The phylogeny of the tribe Aquilini (eagles with fully feathered tarsi) was investigated using 4.2 kb of DNA sequence of one mito- chondrial (cyt b) and three nuclear loci (RAG-1 coding region, LDH intron 3, and adenylate-kinase intron 5). Phylogenetic signal was highly congruent and complementary between mtDNA and nuclear genes. In addition to single-nucleotide variation, shared deletions in nuclear introns supported one basal and two peripheral clades within the Aquilini. Monophyly of the Aquilini relative to other birds of prey was confirmed. However, all polytypic genera within the tribe, Spizaetus, Aquila, Hieraaetus, turned out to be non-monophyletic. Old World Spizaetus and Stephanoaetus together appear to be the sister group of the rest of the Aquilini. Spiza- stur melanoleucus and Oroaetus isidori axe nested among the New World Spizaetus species and should be merged with that genus. The Old World 'Spizaetus' species should be assigned to the genus Nisaetus (Hodgson, 1836). The sister species of the two spotted eagles (Aquila clanga and Aquila pomarina) is the African Long-crested Eagle (Lophaetus occipitalis). -

Chromosome Painting in Three Species of Buteoninae: a Cytogenetic Signature Reinforces the Monophyly of South American Species
Chromosome Painting in Three Species of Buteoninae: A Cytogenetic Signature Reinforces the Monophyly of South American Species Edivaldo Herculano C. de Oliveira1,2,3*, Marcella Mergulha˜o Tagliarini4, Michelly S. dos Santos5, Patricia C. M. O’Brien3, Malcolm A. Ferguson-Smith3 1 Laborato´rio de Cultura de Tecidos e Citogene´tica, SAMAM, Instituto Evandro Chagas, Ananindeua, PA, Brazil, 2 Faculdade de Cieˆncias Exatas e Naturais, ICEN, Universidade Federal do Para´, Bele´m, PA, Brazil, 3 Cambridge Resource Centre for Comparative Genomics, Cambridge, United Kingdom, 4 Programa de Po´s Graduac¸a˜oem Neurocieˆncias e Biologia Celular, ICB, Universidade Federal do Para´, Bele´m, PA, Brazil, 5 PIBIC – Universidade Federal do Para´, Bele´m, PA, Brazil Abstract Buteoninae (Falconiformes, Accipitridae) consist of the widely distributed genus Buteo, and several closely related species in a group called ‘‘sub-buteonine hawks’’, such as Buteogallus, Parabuteo, Asturina, Leucopternis and Busarellus, with unsolved phylogenetic relationships. Diploid number ranges between 2n = 66 and 2n = 68. Only one species, L. albicollis had its karyotype analyzed by molecular cytogenetics. The aim of this study was to present chromosomal analysis of three species of Buteoninae: Rupornis magnirostris, Asturina nitida and Buteogallus meridionallis using fluorescence in situ hybridization (FISH) experiments with telomeric and rDNA probes, as well as whole chromosome probes derived from Gallus gallus and Leucopternis albicollis. The three species analyzed herein showed similar karyotypes, with 2n = 68. Telomeric probes showed some interstitial telomeric sequences, which could be resulted by fusion processes occurred in the chromosomal evolution of the group, including the one found in the tassociation GGA1p/GGA6. -

Studies of Less Familiar Birds Ij2 Lesser Spotted Eagle B.-U
Studies of less familiar birds ij2 Lesser Spotted Eagle B.-U. Meyburg Plates 61-64. Of all European eagles, the Lesser Spotted Eagle Aquila pomarina has the smallest world breeding range, the nominate race being confined to eastern parts of Germany, Poland, eastern Czechoslovakia, Hun gary, Yugoslavia, Romania, Bulgaria, north-east Greece, western Turkey (Thrace), and the Soviet Union north to Leningrad and east to about 35°E. There are no published records of proved breeding in eastern Austria this century, though pairs from Hungary hunt over the land around Lake Neusiedl. There is no clear information on the current position in the Caucasus and the south Caspian lowlands. In Germany the breeding area stretches only to the north of Berlin, westwards beyond the rivers Oder and Neisse but stopping short of the Elbe. In 1969 there were 53 known broods in this area, and possibly a further nine (H. Weber in Glutz von Blotzheim et al. 1971). Even at the beginning of this century, the breeding range extended much further westwards, at least as far as the River Weser in Niedersachsen. A second subspecies, A. p. hastata, breeds in parts of India, in particular the Ganges Valley and West Bengal, and also in Bangla Desh. Hardly anything is known about this form, which appears to be rare. The first autumn plumage is said to be quite different from that of the nominate race, which has led some authors to treat it as a separate species. The Lesser Spotted Eagle presents a very difficult problem to field and museum ornithologists alike—the clear differentiation between it and the very closely related Spotted Eagle A. -

Bald Eagle Haliaeetus Leucocephalus
Wyoming Species Account Bald Eagle Haliaeetus leucocephalus REGULATORY STATUS USFWS: Delisted; Migratory Bird USFS R2: Sensitive USFS R4: Sensitive Wyoming BLM: Sensitive State of Wyoming: Protected Bird CONSERVATION RANKS USFWS: Bird of Conservation Concern WGFD: NSS3 (Bb), Tier II WYNDD: G5, S4B/S5N Wyoming Contribution: LOW IUCN: Least Concern PIF Continental Concern Score: 9 STATUS AND RANK COMMENTS Bald Eagle (Haliaeetus leucocephalus) is provided international protection under the Federal Migratory Bird Treaty Act of 1918, as amended 1. In 1940, Bald Eagle was provided protection under the Bald and Golden Eagle Protection Act 2. In 1966, the southern subspecies was listed as federally endangered under the Endangered Species Preservation Act; the entire population in the contiguous United States was listed as endangered in 1978 under the 1973 Endangered Species Act (ESA). A significant increase in numbers of nesting pairs, productivity, and distribution allowed Bald Eagle to be reclassified from Endangered to Threatened in 1995 under the ESA 3. Bald Eagle was delisted in 2007, and numbers are considered to be stable to increasing across its range 4. The species has been assigned different state conservation ranks by the Wyoming Natural Diversity Database for the breeding season and nonbreeding season because the abundance of the species is different between seasons. NATURAL HISTORY Taxonomy: Bald Eagle is a member of the family Accipitridae, which includes kites, eagles, harriers, and hawks 5. There are two subspecies of Bald Eagle; H. l. alascanus is found north of 40 degrees latitude across North America, including Wyoming, while H. l. leucocephalus is found south of 40 degrees latitude in the Gulf coast states 6. -

RECENT LITERATURE Reviews by Donald S
Vo,.1944xv RecentLiterature [161 raisedand the parents,one or both,were observedmany times carryingfood to the young. In October I securedthe nest for close examinationand still have it. This is the firsttime in the yearsthe HouseWrens have lived on my placethat they have usedthe nest of any other bird or, in fact, built in any but one of my nesting boxes.-•LAUREI•CEB. FLETCHER,Cohasset, Massachusetts. Eastern Goldfinch Makes an 800-Mile Trip.-•A male Goldfinch banded at Ardmore, Pa., by Horace Groskin on April 6, 1942, •vas found dead July, 1943, near St. Andrews,New Brunswick.---HoRacEGRoss:•, 210 Glenn Road, Ardmore, Pennsylvania. RECENT LITERATURE Reviews by Donald S. Farner BANDING 1. Migration of the Red,head from the Utah Breeding Grounds. Cecil Williams. 1944. The Auk, 61(2): 251-259. Of 2,332 young Redheads(Nyroca americana (Eyton)), banded in northern Utah in 1929, 1930 and 1931 there were 357 returns, all shot by sportsmen. The northward dispersionin fall is well illus- trated by September and October returns from Montana, Wyoming, North Dakota, South Dakota and Idaho. Although migration may begin early, many young remain in the breeding grounds until October. The returns show the principal wintering grounds for the Utah birds to be the Salton Sea region of southernCalifornia and the lower coast of Texas from Corpus Christi to Mexico. Eighty-sevenpercent of the birds taken were less than one year old; 10.6 percent were second-yearbirds, and two percent were third year or older. This ratio prompts the author to assumelogically that the first year is the critical one in the life of the bird insofar as shooting is concerned. -

Status of the Eastern Imperial Eagle (Aquila Heliaca) in the European Part of Turkey
ACTA ZOOLOGICA BULGARICA Acta zool. bulg., Suppl. 3, 2011: 87-93 Status of the Eastern Imperial Eagle (Aquila heliaca) in the European part of Turkey Dimitar A. Demerdzhiev1, Stoycho A. Stoychev2, Nikolay G. Terziev2, and Ivaylo D. Angelov2 1 31 Bulgaria Blv�., 4230 Asenovgra�, Bulgaria; E�mails: �emer�jiev@yahoo.�om; �_�emer�[email protected]; w��.bspb.org 2 Haskovo 6300, P.O.Box 130, Bulgaria; E�mails: stoy�hev.s@gmail.�om; w��.bspb.org; [email protected]; ivailoange� [email protected]; w��.bspb.org Abstract: This arti�le presents the results of the �rst more �etaile� stu�ying on the �istribution an� numbers of the Eastern Imperial Eagle (Аquila heliaca SA V I G NY 1809) population in the European part of Turkey. T�enty territories o��upie� by Imperial Eagle pairs, �istribute� in three �ifferent regions �ere �is�overe� �uring the perio� 2008�2009. The bree�ing population was estimate� at 30�50 pairs. The stu�y i�enti�e� t�o main habitat types typi�al of the Imperial Eagles in European Turkey – open hilly areas an� lo� mountain areas (up to 450 m a.s.l.) an� lo� relief plain areas (50�150 m a.s.l.). Poplar trees (Populus sp. L) were i�enti�e� as the most preferre� nesting substratum (44%), follo�e� by Oaks (Quercus sp. L) (40%). Bree�ing �ensity �as 1 pair/100 km2 in both habitat types. The shortest �istan�e bet�een t�o bree�ing pairs �as 5.8 km re�or�e� in plain areas in the Thra�e region. -

Birds of Prey (Accipitriformes and Falconiformes) of Serra De Itabaiana National Park, Northeastern Brazil
Acta Brasiliensis 4(3): 156-160, 2020 Original Article http://revistas.ufcg.edu.br/ActaBra http://dx.doi.org/10.22571/2526-4338416 Birds of prey (Accipitriformes and Falconiformes) of Serra de Itabaiana National Park, Northeastern Brazil Cleverton da Silvaa* i , Cristiano Schetini de Azevedob i , Juan Ruiz-Esparzac i , Adauto de Souza d i Ribeiro h a Programa de Pós-Graduação em Desenvolvimento e Meio Ambiente, Universidade Federal de Sergipe, Aracajú, São Cristóvão, 49100-100, Sergipe, Brasil. *[email protected] b Programa de Pós-Graduação em Ecologia de Biomas Tropicais, Universidade Federal de Ouro Preto, Ouro Preto, 35400-000, Minas Gerais, Brasil. c Universidade Federal de Sergipe, Nossa Senhora da Glória, 49680-000, Sergipe, Brasil. d Universidade Federal de Sergipe, Aracajú, São Cristóvão, 49100-100, Sergipe, Brasil. Received: June 20, 2020 / Accepted: August 27, 2020/ Published online: September 28, 2020 Abstract Birds of prey are important for maintaining ecosystems, since they can regulate the populations of vertebrates and invertebrates. However, anthropic activities, like habitat fragmentation, have been decreasing the number of birds of prey, affecting the habitat ecological relations and, decreasing biodiversity. Our objective was to evaluate species of birds of prey (Accipitriformes and Falconiformes) in a protected area of the Atlantic forest in northeastern Brazil. The area was sampled for 17 months using fixed points and walking along a pre-existing trail. Birds of prey were classified by their Punctual Abundance Index, threat status and forest dependence. Sixteen birds of prey were recorded, being the most common Rupornis magnirostris and Caracara plancus. Most species were considered rare in the area and not dependent of forest vegetation. -

An Early Pleistocene Eagle from Nebraska
248 SHORT COMMUNICATIONS kunthii blossoms by Bombus queens occurred and depend on many factors. That hummingbirds and the workers were unable to secure nectar while positioned ancestor of P. kunthii co-existed may be assumed; within the floral tube, probably as much as lo-20 otherwise its adaptation to hummingbird pollination per cent more nectar was available to Bombus p&her would make little sense. Thus it is possible that P. and Bombus trinominatus populations during this kunthii could have undergone much of its development period due to the feeding activity of Diglossa. under selective pressure from hummingbirds; still, it is clear that Diglossa baritula has co-existed with DISCUSSION AND CONCLUSIONS hummingbirds throughout New World montane hab- Grant and Grant (1968) have proposed an explanation itats for some time and therefore an earlier and more for the reciprocal evolution of hummingbirds and the important role in the evolution of P. kunthii would plants upon which they feed. According to this inter- not be unexpected. This is not to suggest that exploita- pretation most hummingbird-pollinated flowers, espe- tion late in the evolutionary development of P. kunthii cially temperate species, have evolved from bee flowers would be insignificant. Even at present, given the (Grant 1961; Grant and Grant 1965). The process potential counter-selection pressures on P. kunthii involves an incipient stage during which a primitive from bees, the presence of Dglossa perforations un- hummingbird or progenitor already “preadapted” to doubtedly precludes a certain amount of bee pollina- feed on a particular bee flower (in the sense of tion which would probably otherwise occur, helping securing insects within the corolla, or nectar, or both), to maintain the selection pressures on P. -

DRIES ENGELEN - [email protected]
Accounting for differential migration strategies between age groups to monitor raptor population dynamics in the eastern Black Sea flyway (Vansteelant et al. In 2nd review IBIS) DRIES ENGELEN - [email protected] Photo: Adrien Brun One of the world’s largest bottlenecks for raptor migration Based on ‘Raptors of the World’ (Bildstein, 2000) Photo: Adrien Brun Targeted monitoring Priority species, secondary species Using morphological groups MonPalHen, Large Eagle, etc. Quantity vs quality Ageing (& sexing) Photo: John Wright Photo: Adrien Brun Estimated from unidentified Targeted monitoring individuals (%) Species Priority species, secondary species Avg SD Montagu’s Harrier 55,7 10,7 Pallid Harrier 50,5 11,1 Using morphological groups Western Marsh Harrier 0,2 0,1 MonPalHen, Large Eagle, etc. Black Kite 10,0 5,0 Barely mentioned (recorded?) in literature European Honey Buzzard 1,8 1,3 Booted Eagle 0,0 0,0 Quantity vs quality Short-toed Snake Eagle 0,0 0,0 Ageing (& sexing) Lesser Spotted Eagle 43,9 15,6 Photo: Adrien Brun Estimated from unidentified Targeted monitoring individuals (%) Species Priority species, secondary species Avg SD Montagu’s Harrier 55,7 10,7 Pallid Harrier 50,5 11,1 Using morphological groups Western Marsh Harrier 0,2 0,1 MonPalHen, Large Eagle, etc. Black Kite 10,0 5,0 European Honey Buzzard 1,8 1,3 Booted Eagle 0,0 0,0 Quantity vs quality Short-toed Snake Eagle 0,0 0,0 Ageing (& sexing) Lesser Spotted Eagle 43,9 Photo: John15,6 Wright Photo: Adrien Brun Age data is barely used in population trend analyses However 1) Inexperienced juveniles often behave differently than experienced conspecifics (timing, route choice, response to environmental change). -

Uncorrected Proof
Policy and Practice UNCORRECTED PROOF BBarney_C011.inddarney_C011.indd 117979 99/13/2008/13/2008 44:11:24:11:24 PPMM UNCORRECTED PROOF BBarney_C011.inddarney_C011.indd 118080 99/13/2008/13/2008 44:11:24:11:24 PPMM Copy edited by Richard Beatty 11 Conservation Values from Falconry Robert E. Kenward Anatrack Ltd and IUCN-SSC European Sustainable Use Specialist Group, Wareham, UK Introduction Falconry is a type of recreational hunting. This chapter considers the conser- vation issues surrounding this practice. It provides a historical background and then discusses how falconry’s role in conservation has developed and how it could grow in the future. Falconry, as defi ned by the International Association for Falconry and Conservation of Birds of Prey (IAF), is the hunting art of taking quarry in its natural state and habitat with birds of prey. Species commonly used for hunt- ing include eagles of the genera Aquila and Hieraëtus, other ‘broad-winged’ members of the Accipitrinae including the more aggressive buzzards and their relatives, ‘short-winged’ hawks of the genus Accipiter and ‘long-winged’ falcons (genus Falco). Falconers occur in more than 60 countries worldwide, mostly in North America, the Middle East, Europe, Central Asia, Japan and southern Africa. Of these countries, 48 are members of the IAF. In the European Union falconry Recreational Hunting, Conservation and Rural Livelihoods: Science and Practice, 1st edition. Edited by B. Dickson, J. Hutton and B. Adams. © 2009 Blackwell Publishing, UNCORRECTEDISBN 978-1-4051-6785-7 (pb) and 978-1-4051-9142-5 (hb). PROOF BBarney_C011.inddarney_C011.indd 118181 99/13/2008/13/2008 44:11:24:11:24 PPMM Copy edited by Richard Beatty 182 ROBERT E. -
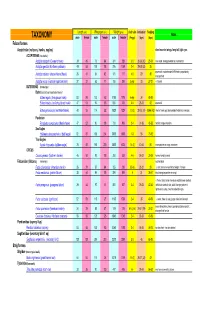
Avian Taxonomy
Length (cm) Wing span (cm) Weight (gms) cluch size incubation fledging Notes TAXONOMY male female male female male female (# eggs) (days) (days) Falconiformes Accipitridae (vultures, hawks, eagles) short rounded wings; long tail; light eyes ACCIPITRINAE (true hawks) Accipiter cooperii (Cooper's hawk) 39 45 73 84 341 528 3-5 30-36 (30) 25-34 crow sized; strongly banded tail; rounded tail Accipiter gentilis (Northern goshawk) 49 58 101 108 816 1059 2-4 28-38 (33) 35 square tail; most abundant NAM hawk; proportionaly 26 31 54 62 101 177 4-5 29 30 Accipiter striatus (sharp-shinned hawk) strongest foot Accipiter nisus (Eurasian sparrowhawk ) 37 37 62 77 150 290 5-Apr 33 27-31 Musket BUTEONINAE (broadwings) Buteo (buzzards or broad tailed hawks) Buteo regalis (ferruginous hawk) 53 59 132 143 1180 1578 6-Apr 34 45-50 Buteo lineatus (red-shouldered hawk) 47 53 96 105 550 700 3-4 28-33 42 square tail Buteo jamaicensis (red-tailed hawk) 48 55 114 122 1028 1224 1-3 (3) 28-35 (34) 42-46 (42) (Harlan' hawk spp); dark patagial featehres: immature; Parabuteo Parabuteo cuncincutus (Harris hawk) 47 52 90 108 710 890 2-4 31-36 45-50 reddish orange shoulders Sea Eagles Haliaeetus leucocephalus (bald eagle) 82 87 185 244 3000 6300 1-3 35 70-92 True Eagles Aquila chrysaetos (golden eagle) 78 82 185 220 3000 6125 1-4 (2) 40-45 50 white patches on wings: immature; CIRCUS Circus cyaneus (Northern harrier) 46 50 93 108 350 530 4-6 26-32 30-35 hovers; hunts by sound Falconidae (falcons) (longwings) notched beak Falco columbarius (American merlin) 26 29 57 64