2017 Flight Course Manual
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

1 ACADEMIC CURRICULUM VITA Jenny Grace Alderden 10 S 2000 E
ACADEMIC CURRICULUM VITA Jenny Grace Alderden 10 S 2000 E Salt Lake City, UT 84112 [email protected] A. EDUCATION Year Degree Institution (Area of Study / Specialization) 2017 PhD University of Utah College of Nursing 2015 Graduate Certificate University of Utah College of Nursing (Teaching Nursing) 2009 MN University of Washington College of Nursing 2001 BSN University of Illinois at Chicago College of Nursing Licensure / Certification 2003– present Critical Care Nurse Specialist, American Association of Critical Care Nurses 2010– present Critical Care Registered Nurse, American Association of Critical Care Nurses Current Registered Nurse, Idaho: N-38675 Current Advanced practice registered nurse (APRN), Idaho: CNS78 B. EMPLOYMENT / PROFESSIONAL EXPERIENCE Dates Position and Institution 07/2019– present Assistant Professor, University of Utah College of Nursing 09/2009—03/2021 Critical Care Nurse Specialist, St. Luke’s Health System 05/2017–07/2019 Assistant Professor, Boise State University School of Nursing 05/2017–07/2019 Adjunct Assistant Professor, University of Utah College of Nursing 06/2009–present Critical Care Nurse Specialist, St. Luke’s Medical Center 09/2009–01/2011 Clinical Instructor/Adjunct Faculty, Boise State University School of Nursing 05/2007–05/2009 Staff Nurse and Charge Nurse, Critical Care Unit, Harrison Medical Center 2007–2009 Captain, 446 Aeromedical Staging Squadron, United States Air Force Reserve 2006–2007 Helicopter Flight Nurse & Shock Trauma Platoon Head Nurse, U.S. Navy Nurse Corps, Al Anbar, Iraq 2005–2006 Team Member, Fixed-Wing Air Transport, Critical Care Air Transport Team, Okinawa, Japan 2005–2006 Lead Nurse, Training/Education, Naval Hospital, Critical Care Unit, Okinawa, Japan 2005–2006 Charge Nurse and Relief Division Officer, Naval Hospital, Critical Care Unit, Okinawa, Japan 2003–2005 Staff Nurse and Charge Nurse, Naval Medical Center, Critical Care Unit, San Diego, CA 2001–2002 Staff Nurse and Charge Nurse, Naval Medical Center, Medical–Surgical Unit, San Diego, CA 1 C. -

What Nurses Need to Know About Informatics, Social Media and Security! – Page 6
FALL 2017 VOLUME 14 {NO1} EDITION 40 www.ncbon.com NURSING BBULLETINULLETIN What Nurses Need to Know about Informatics, Social Media and Security! – page 6 Publication of the North Carolina State Board of Nursing . FALL. 2017 . BULLETIN. N NC BOARD OF NURSING Nursing Bulletin is the official C publication of the North Table of Carolina Board of Nursing. Office Location CONTENTS 4516 Lake Boone Trail Raleigh, NC 27607 VOLUME 14 {NO 1} EDITION 40 Mailing Address P.O. Box 2129 6 What Nurses Need to Know about Raleigh, NC 27602 Informatics, Social Media, and Security! Telephone (919) 782-3211 Substance Use Disorder: Fax 12 (919) 781-9461 Timely Information for Your Practice Website www.ncbon.com 14 Updated Legislation Provides Benefit to Active Duty Office Hours Military & Spouses 8 a.m. to 5 p.m., Monday through Friday 15 NCBON Staff Nationally & Regionally Recognized Board Chair Pat Campbell The Enhanced Nurse Licensure Compact (eNLC): Chief Executive Officer 16 Julia L. George, RN, MSN, FRE Unlocking Access to Nursing Care Across the Nation Editor David Kalbacker 20 Role of the Registered Nurse in North Carolina— Managing Editor Is It Limited? Elizabeth Langdon Mission Statement 26 NCBON Nurse Gateway—Update Your Information The mission of the North Carolina Board of Nursing is to protect the public by regulating the 27 Tribute to Duke Life Flight Team practice of nursing. 28 CE Opportunities 2018 Advertisements contained herein are not necessarily endorsed by the North Carolina Board of 29 Nomination Form Nursing. The publisher reserves the right to accept or reject advertise- ments for the Nursing Bulletin. -

Unit 5 Interactive Notebook Gas Laws and Kinetic Molecular Theory Grant Union High School January 6, 2014 – January 29, 2014
Unit 5 Interactive Notebook Gas Laws and Kinetic Molecular Theory Grant Union High School January 6, 2014 – January 29, 2014 Student Mastery Scale of Learning Goals Date Page Std Learning Goal Homework Mastery 1/6/14 1/7/14 1/8/14 1/9/14 1/10/14 1/13/14 1/14/14 1/15/14 1 1/16/14 1/21/14 1/22/14 1/23/14 1/24/14 1/27/14 1/28/14 1/29/14 Unit 5 EXAM 2 California Standard Gas Laws 4. The kinetic molecular theory describes the motion of atoms and molecules and explains the properties of gases. As a basis for understanding this concept: a. Students know the random motion of molecules and their collisions with a surface create the observable pressure on that surface. Fluids, gases or liquids, consist of molecules that freely move past each other in random directions. Intermolecular forces hold the atoms or molecules in liquids close to each other. Gases consist of tiny particles, either atoms or molecules, spaced far apart from each other and free to move at high speeds. Pressure is defined as force per unit area. The force in fluids comes from collisions of atoms or molecules with the walls of a container. Air pressure is created by the weight of the gas in the atmosphere striking surfaces. Gravity pulls air molecules toward Earth, the surface that they strike. Water pressure can be understood in the same fashion, but the pressures are much greater because of the greater density of water. -

JOB DESCRIPTION: Air Crew / Flight Crew Flight Nurse
JOB DESCRIPTION: Air Crew / Flight Crew Flight Nurse A Career as a MedEvac flight nurse Introduction The Flight Nurse functions as a member of the critical care transport team and is responsible for the care of critically ill or injured patients transported by the STAT MedEvac system by supporting and upholding the mission, goals, and objectives at all times. As the Flight Nurse your responsibilities will include patient care incorporating assessment, stabilization, and intervention techniques consistent with standards and protocols approved by the Medical Director. You will also be expected to execute independent judgment in order to deliver appropriate care that is consistent with the clinical protocols, when contact with a medical command physician is not possible. Duties: Responsible for advanced emergency and critical care patient management during air and ground transport of the critically ill or injured patient from the scene or outlying sites to participating secondary and tertiary care institutions. Provides intensive care monitoring, critical care intervention and advanced surgical skill procedures to critically ill and injured patients that include neonates through geriatric age groups. This includes the management of high-risk obstetrical, medical, surgical, respiratory and cardiovascular patients. The specialized training of the Flight Nurse Specialist allows for the provision of care to critically ill and injured patients through the use of ventilatory support, continuous cardiac and hemodynamic monitoring, including intra-aortic balloon and other cardiac assist devices. Airway management and surgical skill intervention includes cricothyroidotomy, needle chest decompression, chest tube insertion, femoral line insertion and/or cut downs and various intubation techniques. Required Qualifications: Licensed to practice professional nursing. -

18-08 Flight Nurse.Xlsx
TRADITIONAL GUARD OFFICER VACANCY ANNOUNCEMENT NEW YORK AIR NATIONAL GUARD ANNOUNCEMENT NO: 18-24 AIR NATIONAL GUARD BASE 109th Airlift Wing DATE: 27-Aug-18 Stratton Air National Guard Base Scotia, New York 12302-9752 CLOSING DATE: 26-Oct-18 UNIT: AFSC: 46F3 139th Aeromedical Evacuation Squadron Stratton ANGB POSITION TITLE: Flight Nurse Scotia, NY 12302-9752 MAX AVAILABLE GRADE: AREA OF CONSIDERATION: Nationwide Maj. / O-4 All applicants may apply who meet the basic qualifications for this New commissioning Opportunity position and who are eligible for membership in the NYANG. SPECIALTY SUMMARY Provides professional medical-surgical nursing care within scope of practice, established standards of care and federal/ state law. Provides comprehensive nursing care for patients during aeromedical evacuation (AE) flights. Coordinates with and makes recommendations to staff agencies concerning clinical care requirements and medical supplies and equipment required for patient care, AE policies, plans and programs. Supports clinical and operational research activities. DUTIES AND RESPONSIBILITIES Member of the AE crew. Functions as the senior medical member to lead both clinical and aircrew operations of the AE crew during intra-theater, and inter-theater flights. Collaborates with appropriate agencies and personnel to ensure appropriate clinical hand- offs, patient care, planning, and mission success. Directs medical specialty teams (i.e. Critical Care Air Transport, Tactical Critical Care Evacuation, Lung) in the inflight environment. Plans and prepares for AE missions. Coordinates clinical operations with operational aircrew during mission pre-planning. Prepares patient positioning plan to facilitate patient enplaning, nursing care, comfort, and safety. Evaluates individual patient's in- flight needs and requests appropriate medications, supplies, and equipment. -

Air Ambulance Nurses As Expert Supplement to Local Emergency Services Torben Wisborg, MD, Phd,1-3 and Bjørn Bjerkan, Bsc, CRNA2,3
ORIGINAL RESEARCH Air Ambulance Nurses as Expert Supplement to Local Emergency Services Torben Wisborg, MD, PhD,1-3 and Bjørn Bjerkan, BSc, CRNA2,3 Abstract 2 Objective: Flight nurses in the Norwegian National Air well-developed national air ambulance service. The Ambulance Service are specialist nurse anesthetists or intensive Norwegian National Air Ambulance Service has ambulance care nursing specialists. For air ambulance bases far from hospitals, helicopters and dedicated ambulance fixed-wing aircraft dis- tributed over the country, with most fixed-wing aircraft in the these nurses present otherwise unavailable competencies. This northern part where the population density is low. The infra- study reports a 6-year experience with flight nurse participation in structure in Northern Norway was developed through the local emergencies beyond the transportation phase. 1970s by establishing a number of short runway air fields to Methods: The fixed-wing air ambulance base in Alta, Northern enable communications, including patient transport, which Norway (20,000 inhabitants), with 2 aircraft and 2 on-call teams previously was performed by boats and seaplanes. is 150 km by road from the nearest hospital. We did a prospec- In the northernmost part of Norway (Finnmark County), the tive registration of all emergency nonflight missions near the community of Alta is the most inhabited area with approximately air ambulance base from January 1, 2005, to December 31, 20,000 residents (Fig. 1). This population is 150 km by road 2010. from the nearest hospital. The primary health care service in Alta Results: The 217 completed missions corresponded to 3 missions has developed a unique model of a decentralized, advanced per month, half during daytime. -

Vita Nicole A. Underdahl, BSN, RN, CRNA 2010
Vita Nicole A. Underdahl, BSN, RN, CRNA 2010 CRNA 7301 87 th St. NW Trinity Health Burlington, ND 58722 PO Box 5020 Home Tel: 701.725.4395 Minot, ND 58702-5020 Email: [email protected] Tel: 701.857.5000 EDUCATION Master of Science Degree in Nursing Anesthesia Specialty, University of North Dakota, Grand Forks, ND. August, 2009. Bachelor of Science Degree in Nursing, MedCenter One College of Nursing, Bismarck, ND. May, 1996. PRESENTATIONS Underdahl, N., Kelly, K. (November 2004). All MI’s Are the Same…Aren’t They? Trinity Hospital, Minot, ND Underdahl, N., Kelly, K. (January 2005). All MI’s Are the Same…Aren’t They? Part II. Trinity Hospital, Minot, ND Underdahl, N. (April 2009). Indications, Complications, and Techniques for Emergent Rapid Sequence Intubation outside the Operating Room. North Dakota Association of Nurse Anesthetists (NDANA), Fargo, ND. HONORS AND AWARDS Critical Care Registered Nurse (CCRN). 2001-2009 Clinical Ladder through Trinity Health, Minot, ND. 1999-2002. Advanced Clinical Ladder through Trinity Health, Minot, ND. 2002-2007. PROFESSIONAL DEVELOPMENT Service Past Secretary of the American Association of Critical Care Nursing (Roughrider Chapter) 2001- 2002 1 University of North Dakota Class of 2009 Student Representative, serving as a representative for UND on the board of NDANA Affiliations Roughrider Association of Critical Care Nursing American Association for Critical Care Nursing North Dakota Association of Nurse Anesthetists American Association of Nurse Anesthetists PREVIOUS WORK EXPERIENCE 1995-96 Trinity Health . Student Nurse/Nurse Aid. 1996-1998 Trinity Health. Registered Nurse on Surgical floor. 1998-2007 Trinity Health. Intensive Care Unit Registered Nurse, charge nurse, NorthStar Criticair flight nurse. -
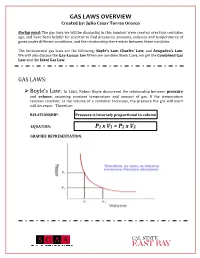
GAS LAWS OVERVIEW Created By: Julio Cesar Torres Orozco
GAS LAWS OVERVIEW Created by: Julio Cesar Torres Orozco Background: The gas laws we will be discussing in this handout were created over four centuries ago, and have been helpful for scientist to find pressures, amounts, volumes and temperatures of gases under different conditions, and the relationship there exists between these variables. The fundamental gas laws are the following: Boyle’s Law, Charles’ Law, and Avogadro’s Law. We will also discuss the Gay-Lussac law When we combine these Laws, we get the Combined Gas Law and the Ideal Gas Law. GAS LAWS: ! Boyle’s Law: In 1662, Robert Boyle discovered the relationship between pressure and volume, assuming constant temperature and amount of gas. If the temperature remains constant, as the volume of a container increases, the pressure the gas will exert will decrease. Therefore: RELATIONSHIP: Pressure is inversely proportional to volume EQUATION: P1 x V1 = P2 x V2 GRAPHIC REPRESENTATION: GAS LAWS OVERVIEW Created by: Julio Cesar Torres Orozco ! Charles’ Law: In 1787, Jacques Charles discovered how temperature and volume are related, assuming that the amount of gas and pressure are constant. An increase in temperature will also increase the volume of the gas. Therefore: RELATIONSHIP: Volume is directly proportional to temperature EQUATION: GRAPHIC REPRESENTATION: ! Gay-Lussacs’ Law: This law shows the relationship there exists between temperature and pressure of gasses. Given a constant volume, if the temperature increases, the pressure will also increase. Therefore: RELATIONSHIP: Pressure is directly proportional to temperature EQUATION: GAS LAWS OVERVIEW Created by: Julio Cesar Torres Orozco GRAPHIC REPRESENTATION: ! Avogadro’s Law: In 1811, Amedeo Avogadro was able to identify the correlation between the amount of gas (n) and its volume, assuming that temperature and pressure are constant. -

The Gas Laws A. Introduction. 1. Comparison of Three States of Matter
CHAPTER FIVE: THE GASEOUS STATE Part One: The Gas Laws A. Introduction. 1. Comparison of three states of matter: solids condensed states liquids (high density, hard to compress) fluids (flow freely) gases (low density, easy to compress) 1 mole liquid H2O occupies 18 mL 1 mole H2O vapor at 100° C and atmospheric pressure occupies 30,600mL Thus, gas molecules must be far apart compared to molecular sizes and interact only weakly. 2. Composition of dry air by volume: 78% N2, 21% O2, 1% Ar, traces of other. 3. Properties of gases: a. Easily compressed into small volumes by applying pressure. b. Exert a pressure P on their surroundings; an equal pressure must be applied to confine them. c. Expand without limit to uniformly and completely occupy the volume of any container. d. Individual molecules exhibit a chaotic motion called diffusion. e. Properties described by gas laws. Show them “A Little Box of Air.” Chapter 5 Page 1 B. Pressure (P). (Section 5.1) 1. P = force per unit area produced by incessant collisions of particles with container walls. 2. Measurement of atmospheric pressure (Torricelli barometer): h ∝ pressure average height h: = 760 mm Hg at sea level P = 760 mm Hg = 1 atmosphere (atm) ≈ 30 inches 1 mm Hg = 1 “torr” SI unit of P is the pascal (Pa) 760 mm Hg: = 1 atm = 1.01325 x 105 Pa = 101.325kPa 3. Pressure of a column of liquid = hydrostatic pressure: P = gdh = accel. of gravity x density of liquid x height of column 2 g=9.81 m/s Chapter 5 Page 2 4. -

Chapter 14: Gases
418-451_Ch14-866418 5/9/06 6:19 PM Page 418 CHAPTER 14 Gases Chemistry 2.a, 3.c, 3.d, 3.e, 4.a, 4.c, 4.d, 4.e, 4.f, 4.g, 4.h I&E 1.b, 1.c, 1.d What You’ll Learn ▲ You will use gas laws to cal- culate how pressure, tem- perature, volume, and number of moles of a gas will change when one or more of these variables is altered. ▲ You will compare properties of real and ideal gases. ▲ You will apply the gas laws and Avogadro’s principle to chemical equations. Why It’s Important From barbecuing on a gas grill to taking a ride in a hot-air balloon, many activities involve gases. It is important to be able to predict what effect changes in pressure, temperature, volume, or amount, will have on the properties and behavior of a gas. Visit the Glencoe Chemistry Web site at chemistrymc.com to find links about gases. Firefighters breathe air that has been compressed into tanks that they can wear on their backs. 418 Chapter 14 418-451_Ch14-866418 5/9/06 6:19 PM Page 419 DISCOVERY LAB More Than Just Hot Air Chemistry 4.a, 4.c I&E 1.d ow does a temperature change affect the air in Ha balloon? Safety Precautions Always wear goggles to protect eyes from broken balloons. Procedure 1. Inflate a round balloon and tie it closed. 2. Fill the bucket about half full of cold water and add ice. 3. Use a string to measure the circumference of the balloon. -

The Ideal and Combined Gas Laws PV = Nrt Or P1V1 = P2V2 T1 T2
The Ideal and Combined Gas Laws PV = nRT or P1V1 = P2V2 T1 T2 Use your knowledge of the ideal and combined gas laws to solve the following problems. If it involves moles or grams, it must be PV = nRT 1) If four moles of a gas at a pressure of 5.4 atmospheres have a volume of 120 liters, what is the temperature? 2) If I initially have a gas with a pressure of 84 kPa and a temperature of 350 C and I heat it an additional 230 degrees, what will the new pressure be? Assume the volume of the container is constant. 3) My car has an internal volume of 2600 liters. If the sun heats my car from a temperature of 200 C to a temperature of 550 C, what will the pressure inside my car be? Assume the pressure was initially 760 mm Hg. 4) How many moles of gas are in my car in problem #3? 5) A toy balloon filled with air has an internal pressure of 1.25 atm and a volume of 2.50 L. If I take the balloon to the bottom of the ocean where the pressure is 95 atmospheres, what will the new volume of the balloon be? How many moles of gas does the balloon hold? (Assume T = 285 K) For chemistry help, visit www.chemfiesta.com © 2000 Cavalcade Publishing - All Rights Reserved MIXED GAS LAWS WORKSHEET Created by Tara L. Moore at www.learning.mgccc.cc.ms.us/pk/sciencedocs/gaslawwksheet.htm Directions: Answer each question below. -

The Effects of Carbonated Beverages on Arterial Oxygen Saturation, Serum Hemoglobin Concentration and Maximal Oxygen Consumption
AN ABSTRACT OF THE THESIS OF Max Waibler for the degree of Master of Science in Human Performance presented on August 21. 1991. Title : The Effects of Carbonated Beverages on Arterial Oxygen Saturation.Serum Hemoglobin Concentration and Maximal Oxygen Consumption Abstract approved :_Redacted for Privacy Dr. AWthony R. Wilcox Elite milers, Sir Roger Bannister and Joseph Falcon, have stated that the consumption of carbonated beverages hinders the performance of aerobic events. Oxygen transport is purportedly impaired by the consumption of carbonated beverages. The research on carbonated beverages has been limited to the effects on the digestive system, gastric emptying, and thermal heat stress in animals. The purpose of this study was to investigate the effects of consuming 28 ounces of carbonated beverages per day, for three weeks,on arterial oxygen saturation (Sa02), serum hemoglobin concentrations (Hb), and maximal oxygen consumption (VO2max) in experienced cyclists. Nine competitive cyclists and triathletes (aged 19-24 years, M= 21.67 years), with average weights and percent body fat of 76.51 kg and 11.4 percent respectively, were randomly assigned to a three week period of consuming 28 ounces of carbonated water or a three week period of no carbonated beverages. At the end of each three week period, a 5 c.c. blood sample was taken for Hb determination and the subjects performed a test of maximal oxygen consumption on a cycle ergometer while Sa02 was being monitored. The groups then crossed-over with respect to their treatment, and after another three week period, the same variables were measured. The Student's tstatistic was used to compare Sa02, Hb, and VO2max.