And Epoxidesepoxides
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Progression of Chiral Anions from Concepts to Applications in Asymmetric Catalysis Robert J
REVIEW ARTICLE PUBLISHED ONLINE: 24 JULY 2012 | DOI: 10.1038/NCHEM.1405 The progression of chiral anions from concepts to applications in asymmetric catalysis Robert J. Phipps, Gregory L. Hamilton and F. Dean Toste* Despite the tremendous advances of the past four decades, chemists are far from being able to use chiral catalysts to con- trol the stereoselectivity of any desired reaction. New concepts for the construction and mode of operation of chiral catalysts have the potential to open up previously inaccessible reaction space. The recognition and categorization of distinct approaches seems to play a role in triggering rapid exploration of new territory. This Review both reflects on the origins as well as details a selection of the latest examples of an area that has advanced considerably within the past five years or so: the use of chiral anions in asymmetric catalysis. Defining reactions as involving chiral anions is a difficult task owing to uncertainties over the exact catalytic mechanisms. Nevertheless, we attempt to provide an overview of the breadth of reactions that could reasonably fall under this umbrella. 9 ithin the broader field of science, organic chemistry is Of significantly greater acidity (pKa around 2–4) are chiral phos- often referred to as a mature field of study. In spite of this, phoric acids, commonly derived from BINOL (1,1′-bi-2-naphthol). Wnew avenues of inquiry seem to arise with relative fre- These catalysts have risen to prominence over the past decade quency. Perhaps we take it for granted that expansions of the field because their high acidity permits activation of a broad range of are sparked when one or two chemical laboratories uncover a new substrates3,10. -

Organic Chemistry
Wisebridge Learning Systems Organic Chemistry Reaction Mechanisms Pocket-Book WLS www.wisebridgelearning.com © 2006 J S Wetzel LEARNING STRATEGIES CONTENTS ● The key to building intuition is to develop the habit ALKANES of asking how each particular mechanism reflects Thermal Cracking - Pyrolysis . 1 general principles. Look for the concepts behind Combustion . 1 the chemistry to make organic chemistry more co- Free Radical Halogenation. 2 herent and rewarding. ALKENES Electrophilic Addition of HX to Alkenes . 3 ● Acid Catalyzed Hydration of Alkenes . 4 Exothermic reactions tend to follow pathways Electrophilic Addition of Halogens to Alkenes . 5 where like charges can separate or where un- Halohydrin Formation . 6 like charges can come together. When reading Free Radical Addition of HX to Alkenes . 7 organic chemistry mechanisms, keep the elec- Catalytic Hydrogenation of Alkenes. 8 tronegativities of the elements and their valence Oxidation of Alkenes to Vicinal Diols. 9 electron configurations always in your mind. Try Oxidative Cleavage of Alkenes . 10 to nterpret electron movement in terms of energy Ozonolysis of Alkenes . 10 Allylic Halogenation . 11 to make the reactions easier to understand and Oxymercuration-Demercuration . 13 remember. Hydroboration of Alkenes . 14 ALKYNES ● For MCAT preparation, pay special attention to Electrophilic Addition of HX to Alkynes . 15 Hydration of Alkynes. 15 reactions where the product hinges on regio- Free Radical Addition of HX to Alkynes . 16 and stereo-selectivity and reactions involving Electrophilic Halogenation of Alkynes. 16 resonant intermediates, which are special favor- Hydroboration of Alkynes . 17 ites of the test-writers. Catalytic Hydrogenation of Alkynes. 17 Reduction of Alkynes with Alkali Metal/Ammonia . 18 Formation and Use of Acetylide Anion Nucleophiles . -

Aldehydes and Ketones
12 Aldehydes and Ketones Ethanol from alcoholic beverages is first metabolized to acetaldehyde before being broken down further in the body. The reactivity of the carbonyl group of acetaldehyde allows it to bind to proteins in the body, the products of which lead to tissue damage and organ disease. Inset: A model of acetaldehyde. (Novastock/ Stock Connection/Glow Images) KEY QUESTIONS 12.1 What Are Aldehydes and Ketones? 12.8 What Is Keto–Enol Tautomerism? 12.2 How Are Aldehydes and Ketones Named? 12.9 How Are Aldehydes and Ketones Oxidized? 12.3 What Are the Physical Properties of Aldehydes 12.10 How Are Aldehydes and Ketones Reduced? and Ketones? 12.4 What Is the Most Common Reaction Theme of HOW TO Aldehydes and Ketones? 12.1 How to Predict the Product of a Grignard Reaction 12.5 What Are Grignard Reagents, and How Do They 12.2 How to Determine the Reactants Used to React with Aldehydes and Ketones? Synthesize a Hemiacetal or Acetal 12.6 What Are Hemiacetals and Acetals? 12.7 How Do Aldehydes and Ketones React with CHEMICAL CONNECTIONS Ammonia and Amines? 12A A Green Synthesis of Adipic Acid IN THIS AND several of the following chapters, we study the physical and chemical properties of compounds containing the carbonyl group, C O. Because this group is the functional group of aldehydes, ketones, and carboxylic acids and their derivatives, it is one of the most important functional groups in organic chemistry and in the chemistry of biological systems. The chemical properties of the carbonyl group are straightforward, and an understanding of its characteristic reaction themes leads very quickly to an understanding of a wide variety of organic reactions. -

Recent Advances in Titanium Radical Redox Catalysis
JOCSynopsis Cite This: J. Org. Chem. 2019, 84, 14369−14380 pubs.acs.org/joc Recent Advances in Titanium Radical Redox Catalysis Terry McCallum, Xiangyu Wu, and Song Lin* Department of Chemistry and Chemical Biology, Cornell University, Ithaca, New York 14853, United States ABSTRACT: New catalytic strategies that leverage single-electron redox events have provided chemists with useful tools for solving synthetic problems. In this context, Ti offers opportunities that are complementary to late transition metals for reaction discovery. Following foundational work on epoxide reductive functionalization, recent methodological advances have significantly expanded the repertoire of Ti radical chemistry. This Synopsis summarizes recent developments in the burgeoning area of Ti radical catalysis with a focus on innovative catalytic strategies such as radical redox-relay and dual catalysis. 1. INTRODUCTION a green chemistry perspective, the abundance and low toxicity of Ti make its complexes highly attractive as reagents and Radical-based chemistry has long been a cornerstone of 5 1 catalysts in organic synthesis. synthetic organic chemistry. The high reactivity of organic IV/III radicals has made possible myriad new reactions that cannot be A classic example of Ti -mediated reactivity is the reductive ring opening of epoxides. This process preferentially readily achieved using two-electron chemistry. However, the − high reactivity of organic radicals is a double-edged sword, as cleaves and functionalizes the more substituted C O bond, the selectivity of these fleeting intermediates can be difficult to providing complementary regioselectivity to Lewis acid control in the presence of multiple chemotypes. In addition, promoted epoxide reactions. The synthetic value of Ti redox catalysis has been highlighted by their many uses in total catalyst-controlled regio- and stereoselective reactions involv- 6−10 ing free-radical intermediates remain limited,2 and the synthesis (Scheme 1). -

DNA Sequence Selectivity of Guanine-N7 Alkylation by Three Antitumor Chloroethylating Agents
ICANCER RESEARCH 46, 1943-1947, April 19861 DNA Sequence Selectivity of Guanine-N7 Alkylation by Three Antitumor Chloroethylating Agents John A. Hartley,' Neil W. Gibson, Kurt W. Kohn, and William B. Mattes@ L.aboratory ofMolecular Pharmacology. Developmental Therapeutics Program, Division ofCancer Treatment, National Cancer institute, NiH, Bethesda, Maryland 20205 ABSTRACF (4). Both compounds are like C1EtNUs in that they produce The DNA sequenceselectivitiesof guanine-N7alkylation producedby delayed interstrand cross-linking in Mer (guanine-O@-alkyl three chloroethylating antitumor agents, I-(2-chloroethyl)-3-(cis-2-hy transferase deficient) human tumor cell lines, but not in Mer droxy)cyclohexyl-l-nitrosourea(cis-2-OH CCNU), 2-chloroethyl(meth cells, DNA-protein cross-linking in both cell types without ylsulfonyl)methanesulfonate,and 8-carhamoyl-3-(2-chloroethyl)imidazo delay, and selective killing of the Mer cells (5, 6). 15,l-dl-l,2,3,5-tetrazin-4(3H)-one (mitozolomide), were examined using We have recently investigated whether either of these agents a modificationof the Maxam and Gilbert sequencing technique. In a had a simpler alkylation chemistry than the C1EtNUs. One of region ofpBR322 DNA, 2-chloroethyl(methylsulfonyl)methanesulfonate the major alkylation routes of the C1EtNUs is the production produced approximately the same degree of alkylation at all guanines. of hydroxyethyl adducts in DNA (7). Such adducts are thought cis-2-OH CCNU, however,preferentiallyalkylated the middleguanines to have little importance in the expression ofantitumor activity in runs of three or more guanines the intensity of the reaction increased but may contribute to both the mutagenic and carcinogenic with the number of adjacent guanines in the DNA sequence. -
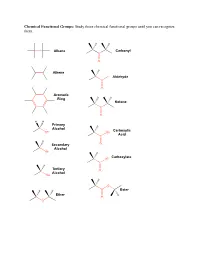
Study These Chemical Functional Groups Until You Can Recognize Them
Chemical Functional Groups: Study these chemical functional groups until you can recognize them. Alkane Carbonyl C O Alkene H Aldehyde C O Aromatic Ring Ketone C O H H Primary Alcohol Carboxylic OH OH Acid C H Secondary O Alcohol OH Carboxylate O C Tertiary O Alcohol OH O Ester Ether O O Primary Primary Ammonium Amine Ion NH3 NH2 Secondary Secondary Ammonium Amine Ion N N H H2 Tertiary Tertiary Ammonium Amine H N N Ion H Amide Imine N N O Thiol Thioester S SH O Sulfide S O Monophosphate P O O O O O Diphosphate P P O O O O O O O O Triphosphate P P P O O O O O O O 1. Draw the chemical structure of each amino acid for yourself. Identify every chemical functional group of each amino acid sidechain. ________________________________________________________________________________________________________ Hydrogen Bond Acceptors/Donors: “Polar molecules containing O-H and N-H bonds and the molecule HF have very large dipole moments and stronger than average dipole-dipole interactions. The hydrogen atoms in these molecules are bonded to small, highly electronegative atoms and have large partial positive charge. Therefore, the unequal distribution of the electrons in these molecules gives rise to large dipole moments. Because of its strength, the interaction between the partially positive hydrogen atom on one molecule and a partially negative O, N, or F atom with unshared pairs of electrons on an adjacent molecule merits special distinction: it is called a hydrogen bond” [Gilbert, Kirss, Foster, and Davies. Chemistry, 2nd Ed. 2009. pp. 473-474] 2. -

Unusual Regioselectivity in the Opening of Epoxides by Carboxylic Acid Enediolates
Molecules 2008, 13, 1303-1311 manuscripts; DOI: 10.3390/molecules13061303 OPEN ACCESS molecules ISSN 1420-3049 www.mdpi.org/molecules Communication Unusual Regioselectivity in the Opening of Epoxides by Carboxylic Acid Enediolates Luis R. Domingo, Salvador Gil, Margarita Parra* and José Segura Department of Organic Chemistry, Universitat de València, Dr. Moliner 50, 46100 Burjassot, Spain. Fax +34(9)63543831; E-mails: [email protected]; [email protected]; [email protected] * Author to whom correspondence should be addressed; E-mail: [email protected] Received: 29 May 2008; in revised form: 5 June 2008 / Accepted: 6 June 2008 / Published: 9 June 2008 Abstract: Addition of carboxylic acid dianions appears to be a potential alternative to the use of aluminium enolates for nucleophilic ring opening of epoxides. These conditions require the use of a sub-stoichiometric amount of amine (10% mol) for dianion generation and the previous activation of the epoxide with LiCl. Yields are good, with high regioselectivity, but the use of styrene oxide led, unexpectedly, to a mixture resulting from the attack on both the primary and secondary carbon atoms. Generally, a low diastereoselectivity is seen on attack at the primary center, however only one diastereoisomer was obtained from attack to the secondary carbon of the styrene oxide. Keywords: Lactones, lithium chloride, nucleophilic addition, regioselectivity, diastereoselectivity. Introduction Epoxides have been recognized among the most versatile compounds in organic synthesis, not only as final products [1] but as key intermediates for further manipulations. Accordingly, new synthetic developments are continuously being published [2]. Due to its high ring strain (around 27 Kcal/mol) its ring-opening, particularly with carbon-based nucleophiles, is a highly valuable synthetic strategy Molecules 2008, 13 1304 [3]. -

Highly Efficient Epoxidation of Olefins with Hydrogen Peroxide Oxidant Using Modified Silver Polyoxometalate Catalysts
African Journal of Pure and Applied Chemistry Vol. 7(2), pp. 50-55, February 2013 Available online at http://www.academicjournals.org/AJPAC DOI: 10.5897/AJPAC12.060 ISSN 1996 - 0840 ©2013 Academic Journals Full Length Research Paper Highly efficient epoxidation of olefins with hydrogen peroxide oxidant using modified silver polyoxometalate catalysts Emmanuel Tebandeke 1*, Henry Ssekaalo 1 and Ola F. Wendt 2 1Department of Chemistry, Makerere University, P. O. Box 7062 Kampala, Uganda. 2Organic Chemistry, Department of Chemistry, Lund University, P. O. Box 124, 221 00 Lund, Sweden. Accepted 31 January, 2013 The catalytic epoxidation of olefins is an important reaction in chemical industry since epoxides are versatile and important intermediates in the synthesis of fine chemicals and pharmaceuticals. The current epoxidation procedures often use stoichiometric organic and sometimes toxic inorganic oxidants that are less favourable from an environmental and economical point of view. In contrast to such processes, catalytic epoxidation processes employing H2O2 oxidant are preferred for green processing. Herein is reported a highly efficient green process for the epoxidation of olefins using H2O2 oxidant and modified silver polyoxometalate catalysts at 65°C. The preparation and activity of the catalysts are described. The method enjoys >90% conversion and ≥99% selectivity to the epoxide for a variety of cyclic and linear olefins including terminal ones. The catalysts are easily recovered by filtration and are reusable several times. Key words: Epoxidation, olefins, hydrogen peroxide, polyoxometalate, silver catalysts. INTRODUCTION The catalytic epoxidation of olefins is very important in compared to organic peroxides and peracids (Jones, the chemical industry since epoxides are versatile and 1999; Lane and Burgess, 2003). -

Part I. the Total Synthesis Of
AN ABSTRACT OF THE THESIS OF Lester Percy Joseph Burton forthe degree of Doctor of Philosophy in Chemistry presentedon March 20, 1981. Title: Part 1 - The Total Synthesis of (±)-Cinnamodialand Related Drimane Sesquiterpenes Part 2 - Photochemical Activation ofthe Carboxyl Group Via NAcy1-2-thionothiazolidines Abstract approved: Redacted for privacy DT. James D. White Part I A total synthesis of the insect antifeedant(±)-cinnamodial ( ) and of the related drimanesesquiterpenes (±)-isodrimenin (67) and (±)-fragrolide (72)are described from the diene diester 49. Hydro- boration of 49 provided the C-6oxygenation and the trans ring junction in the form of alcohol 61. To confirm the stereoselectivity of the hydroboration, 61 was convertedto both (t)-isodrimenin (67) and (±)-fragrolide (72) in 3 steps. A diisobutylaluminum hydride reduction of 61 followed by a pyridiniumchlorochromate oxidation and treatment with lead tetraacetate yielded the dihydrodiacetoxyfuran102. The base induced elimination of acetic acid preceded theepoxidation and provided 106 which contains the desired hydroxy dialdehydefunctionality of cinnamodial in a protected form. The epoxide 106 was opened with methanol to yield the acetal 112. Reduction, hydrolysis and acetylation of 112 yielded (t)- cinnamodial in 19% overall yield. Part II - Various N- acyl- 2- thionothiazolidineswere prepared and photo- lysed in the presence of ethanol to provide the corresponding ethyl esters. The photochemical activation of the carboxyl function via these derivatives appears, for practical purposes, to be restricted tocases where a-keto hydrogen abstraction and subsequent ketene formation is favored by acyl substitution. Part 1 The Total Synthesis of (±)-Cinnamodial and Related Drimane Sesquiterpenes. Part 2 Photochemical Activation of the Carboxyl Group via N-Acy1-2-thionothiazolidines. -

CYCLIC OXONIUM IONS and RELATED STRUCTURES* Whose
CYCLIC OXONIUM IONS AND RELATED STRUCTURES* BY ROBERT J. GARGIULO AND D. STANLEY TARBELL DEPARTMENT OF CHEMISTRY, VANDERBILT UNIVERSITY Communicated October 28, 1968 Abstract and Summary.-This paper describes the preparation by several methods of trialkyloxonium ions containing five-membered rings and the study of their structures by observing the nuclear magnetic resonance (NMR) spectra in several solvents. This provides independent evidence for these compounds, whose existence has been shown by kinetic, preparative, and stereochemical studies. The following SbCl6 oxonium salts have been prepared, isolated, and character- ized as crystalline solids: the 1,2,5-trimethyltetrahydrofuranium (Ha), the 1,2,2,5-tetramethyl (JIb), the trans-l-methylperhydrobenzofuranium (IVa), the trans-1,2-dimethyl (IVb), and the trans-1,2,2-trimethyl (IVc) compounds. The trifluoroacetate salts IIc, IId, IVd, and LWe have been made in acid solution, and all the salts II and IV have been characterized by their NMR spectra as cyclic- oxonium salts, with the charged 1-CH3 group downfield from an uncharged -OCH3. Spectra of all the SbCl6 salts in nitrobenzene solution are reported. Further evidence for the cyclic structure is given by the identification of 2,2,5- trimethyltetrahydrofuran (VIII) from the action of pyridine and lithium chloride on the 1,2,2,5-tetramethyltetrahydrofuranium salt JIb. Solvolysis of this salt by ethanol gave 2-ethoxy-2-methyl-5-methoxyhexane (VII) by attack at the tertiary carbon of the oxonium ion. The structure of VII was established by its synthesis and that of the other possible isomer. The trifluoroacetate oxonium ion (IVe) gave, after three days in 5 per cent sulfuric-trifluoroacetic acid, trans-2,2- dimethylperhydrobenzofuran and methyl trifluoroacetate. -

Epoxidation of Olefins Using Molecular Oxygen
Epoxidation of Olefins Using Molecular Oxygen Zhongxing Huang Sep 16th, 2015 1 Major Challenge in Catalysis Key points . Low temperature . Selective May 31st, 1993, C&EN News. 2 Most Ancient Chemistry-Oxygenase Monooxygenase catalyzes the incorporation of one atom of oxygen into the product Iron-Containing Enzymes, RSC Publishing, 2011. 3 Most Ancient Chemistry-Oxygenase Dioxygenase incorporates both oxygen atoms into the product Shen, B.; Gould, S. J. Biochemistry 1991, 30, 8936. Gould, S. J.; Kirchmeier, M. J.; LaFever, R. E. J. Am. Chem. Soc. 1996, 118, 7663. 4 Most Ancient Chemistry-Oxygenase Monooxygenase catalyzes the incorporation of one atom of oxygen into the product . Ideal system should avoid use of reductants Dioxygenase incorporates both oxygen atoms into the product 5 Most Ancient Chemistry-Oxygenase Monooxygenase catalyzes the incorporation of one atom of oxygen into the product . Ideal system should avoid use of reductants Dioxygenase incorporates both oxygen atoms into the product Ideal Dioxygenase incorporates both oxygen atoms into the epoxide 6 Most Ancient Chemistry-Oxygenase Monooxygenase catalyzes the incorporation of one atom of oxygen into the product . Ideal system should avoid use of reductants Dioxygenase incorporates both oxygen atoms into the product Ideal Dioxygenase incorporates both oxygen atoms into the epoxide 7 Industrial Process- EO and PO . Top chemicals produced in US (2004,103 ton) 1 sulfuric acid 35954 2 nitrogen 30543 3 ethylene 25682 4 oxygen 25568 5 propylene 15345 6 chlorine 12166 7 ethylene dichloride 12163 8 phosphoric acid 11463 9 ammonia 10762 10 sodium hydroxide 9508 11 benzene 7675 12 nitric acid 6703 13 ammonium nitrate 6021 14 ethylbenzene 5779 15 urea 5755 16 styrene 5394 17 hydrochloric acid 5012 18 ethylene oxide 3772 19 cumene 3736 20 ammonium sulfate 2643 8 Industrial Process- EO and PO . -

Tricyclic Oxonium Tamed
RESEARCH HIGHLIGHTS Nature Reviews Chemistry | https://doi.org/10.1038/s41570-019-0150-y | Published online 13 November 2019 NATURAL PRODUCT SYNTHESIS could only be characterized at low temperature. However, 1H and 13C NMR spectra are indicative of its Tricyclic oxonium tamed formation with large downfield shifts of the signals for the three Natural products or secondary formal positive charge on oxygen carbons adjacent to the oxygen and metabolites have hugely diverse (a trialkyloxonium ion) proposed for the protons attached to them. We decided structures. Simple-looking linear for other biosyntheses have proved In addition, a new cross-peak was to try to precursors can, via highly reactive far more elusive. observed in the 1H–13C correlation intermediates, fold up into a variety “The structures of a number spectrum, indicating the formation investigate of complex 3D structures. Writing of Laurencia natural products had of a new transannular bond. 1H and whether these in the Journal of the American originally been misassigned,” explains 13C chemical shifts calculated somewhat Chemical Society, Jonathan Burton Burton. “Our work with Rob Paton on using density functional theory also exotic looking and co-workers from the University calculating the NMR spectra for the favoured the assigned structures and of Oxford, in collaboration with proposed structures of these natural formation of the oxonium ions. species might Robert Paton at Colorado State products led us to earlier proposals of Four different oxonium ions really exist University, describe the synthesis and tricyclic oxonium species. We decided derived from the diastereomers characterization of tricyclic oxonium to try to investigate whether these of laurediol were prepared and intermediates and their conversion somewhat exotic looking species characterized — the formation of into ten different natural products might really exist.” one of these, from 3Z, S,S-laurediol originally isolated from algae of the In the proposed biosyntheses, is pictured.