Bryophytes and Lichens: Small but Indispensable Forest Dwellers
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
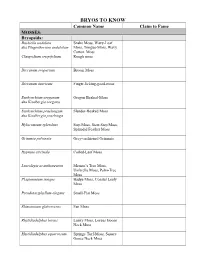
Bryo's to Know Table
BRYOS TO KNOW Common Name Claim to Fame MOSSES: Bryopsida: Buckiella undulata Snake Moss, Wavy-Leaf aka Plagiothecium undulatum Moss, Tongue-Moss, Wavy Cotton, Moss Claopodium crispifolium Rough moss Dicranum scoparium Broom Moss Dicranum tauricum Finger-licking-good-moss Eurhynchium oreganum Oregon Beaked-Moss aka Kindbergia oregana Eurhynchium praelongum Slender-Beaked Moss aka Kindbergia praelonga Hylocomium splendens Step Moss, Stair-Step Moss, Splendid Feather Moss Grimmia pulvinata Grey-cushioned Grimmia Hypnum circinale Coiled-Leaf Moss Leucolepis acanthoneuron Menzie’s Tree Moss, Umbrella Moss, Palm-Tree Moss Plagiomnium insigne Badge Moss, Coastal Leafy Moss Pseudotaxiphyllum elegans Small-Flat Moss Rhizomnium glabrescens Fan Moss Rhytidiadelphus loreus Lanky Moss, Loreus Goose Neck Moss Rhytidiadelphus squarrosum Springy Turf-Moss, Square Goose Neck Moss Rhytidiadelphus triquetrus Electrified Cat-Tail Moss, Goose Necked Moss Rhytidiopsus robusta Robust mountain moss Schistostega pennata Goblin’s Gold, Luminous Moss Polytrichopsida: Atrichum Atrichum Moss , Crane’s Bill Moss (for Atrichum selwynii) Pogonatum contortum Contorted Pogonatum Moss Polytrichum commune Common Hair Cap Moss Polytrichum piliferum Bristly Haircap Moss Andreaeopsida Andreaea nivalis Granite moss, Lantern moss, Snow Rock Moss Sphagnopsida: Sphagnum capillifolium Red Bog Moss, Small Red Peat Moss Sphagnum papillosum Fat Bog Moss, Papillose sphagnum Sphagnum squarrosum Shaggy Sphagnum, Spread- Leaved Peat Moss Takakiopsida: Takakia lepidoziooides Impossible -

Phytotaxa, a Synthesis of Hornwort Diversity
Phytotaxa 9: 150–166 (2010) ISSN 1179-3155 (print edition) www.mapress.com/phytotaxa/ Article PHYTOTAXA Copyright © 2010 • Magnolia Press ISSN 1179-3163 (online edition) A synthesis of hornwort diversity: Patterns, causes and future work JUAN CARLOS VILLARREAL1 , D. CHRISTINE CARGILL2 , ANDERS HAGBORG3 , LARS SÖDERSTRÖM4 & KAREN SUE RENZAGLIA5 1Department of Ecology and Evolutionary Biology, University of Connecticut, 75 North Eagleville Road, Storrs, CT 06269; [email protected] 2Centre for Plant Biodiversity Research, Australian National Herbarium, Australian National Botanic Gardens, GPO Box 1777, Canberra. ACT 2601, Australia; [email protected] 3Department of Botany, The Field Museum, 1400 South Lake Shore Drive, Chicago, IL 60605-2496; [email protected] 4Department of Biology, Norwegian University of Science and Technology, N-7491 Trondheim, Norway; [email protected] 5Department of Plant Biology, Southern Illinois University, Carbondale, IL 62901; [email protected] Abstract Hornworts are the least species-rich bryophyte group, with around 200–250 species worldwide. Despite their low species numbers, hornworts represent a key group for understanding the evolution of plant form because the best–sampled current phylogenies place them as sister to the tracheophytes. Despite their low taxonomic diversity, the group has not been monographed worldwide. There are few well-documented hornwort floras for temperate or tropical areas. Moreover, no species level phylogenies or population studies are available for hornworts. Here we aim at filling some important gaps in hornwort biology and biodiversity. We provide estimates of hornwort species richness worldwide, identifying centers of diversity. We also present two examples of the impact of recent work in elucidating the composition and circumscription of the genera Megaceros and Nothoceros. -

Coptis Trifolia Conservation Assessment
CONSERVATION ASSESSMENT for Coptis trifolia (L.) Salisb. Originally issued as Management Recommendations December 1998 Marty Stein Reconfigured-January 2005 Tracy L. Fuentes USDA Forest Service Region 6 and USDI Bureau of Land Management, Oregon and Washington CONSERVATION ASSESSMENT FOR COPTIS TRIFOLIA Table of Contents Page List of Tables ................................................................................................................................. 2 List of Figures ................................................................................................................................ 2 Summary........................................................................................................................................ 4 I. NATURAL HISTORY............................................................................................................. 6 A. Taxonomy and Nomenclature.......................................................................................... 6 B. Species Description ........................................................................................................... 6 1. Morphology ................................................................................................................... 6 2. Reproductive Biology.................................................................................................... 7 3. Ecological Roles ............................................................................................................. 7 C. Range and Sites -

The Puzzle of Lichen Symbiosis
Digital Comprehensive Summaries of Uppsala Dissertations from the Faculty of Science and Technology 1503 The puzzle of lichen symbiosis Pieces from Thamnolia IOANA ONUT, -BRÄNNSTRÖM ACTA UNIVERSITATIS UPSALIENSIS ISSN 1651-6214 ISBN 978-91-554-9887-0 UPPSALA urn:nbn:se:uu:diva-319639 2017 Dissertation presented at Uppsala University to be publicly examined in Lindhalsalen, EBC, Norbyvägen 14, Uppsala, Thursday, 1 June 2017 at 09:15 for the degree of Doctor of Philosophy. The examination will be conducted in English. Faculty examiner: Associate Professor Anne Pringle (University of Wisconsin-Madison, Department of Botany). Abstract Onuț-Brännström, I. 2017. The puzzle of lichen symbiosis. Pieces from Thamnolia. Digital Comprehensive Summaries of Uppsala Dissertations from the Faculty of Science and Technology 1503. 62 pp. Uppsala: Acta Universitatis Upsaliensis. ISBN 978-91-554-9887-0. Symbiosis brought important evolutionary novelties to life on Earth. Lichens, the symbiotic entities formed by fungi, photosynthetic organisms and bacteria, represent an example of a successful adaptation in surviving hostile environments. Yet many aspects of the lichen symbiosis remain unexplored. This thesis aims at bringing insights into lichen biology and the importance of symbiosis in adaptation. I am using as model system a successful colonizer of tundra and alpine environments, the worm lichens Thamnolia, which seem to only reproduce vegetatively through symbiotic propagules. When the genetic architecture of the mating locus of the symbiotic fungal partner was analyzed with genomic and transcriptomic data, a sexual self-incompatible life style was revealed. However, a screen of the mating types ratios across natural populations detected only one of the mating types, suggesting that Thamnolia has no potential for sexual reproduction because of lack of mating partners. -

Global Biodiversity Patterns of the Photobionts Associated with the Genus Cladonia (Lecanorales, Ascomycota)
Microbial Ecology https://doi.org/10.1007/s00248-020-01633-3 FUNGAL MICROBIOLOGY Global Biodiversity Patterns of the Photobionts Associated with the Genus Cladonia (Lecanorales, Ascomycota) Raquel Pino-Bodas1 & Soili Stenroos2 Received: 19 August 2020 /Accepted: 22 October 2020 # The Author(s) 2020 Abstract The diversity of lichen photobionts is not fully known. We studied here the diversity of the photobionts associated with Cladonia, a sub-cosmopolitan genus ecologically important, whose photobionts belong to the green algae genus Asterochloris. The genetic diversity of Asterochloris was screened by using the ITS rDNA and actin type I regions in 223 specimens and 135 species of Cladonia collected all over the world. These data, added to those available in GenBank, were compiled in a dataset of altogether 545 Asterochloris sequences occurring in 172 species of Cladonia. A high diversity of Asterochloris associated with Cladonia was found. The commonest photobiont lineages associated with this genus are A. glomerata, A. italiana,andA. mediterranea. Analyses of partitioned variation were carried out in order to elucidate the relative influence on the photobiont genetic variation of the following factors: mycobiont identity, geographic distribution, climate, and mycobiont phylogeny. The mycobiont identity and climate were found to be the main drivers for the genetic variation of Asterochloris. The geographical distribution of the different Asterochloris lineages was described. Some lineages showed a clear dominance in one or several climatic regions. In addition, the specificity and the selectivity were studied for 18 species of Cladonia. Potentially specialist and generalist species of Cladonia were identified. A correlation was found between the sexual reproduction frequency of the host and the frequency of certain Asterochloris OTUs. -

Anthocerotophyta
Glime, J. M. 2017. Anthocerotophyta. Chapt. 2-8. In: Glime, J. M. Bryophyte Ecology. Volume 1. Physiological Ecology. Ebook 2-8-1 sponsored by Michigan Technological University and the International Association of Bryologists. Last updated 5 June 2020 and available at <http://digitalcommons.mtu.edu/bryophyte-ecology/>. CHAPTER 2-8 ANTHOCEROTOPHYTA TABLE OF CONTENTS Anthocerotophyta ......................................................................................................................................... 2-8-2 Summary .................................................................................................................................................... 2-8-10 Acknowledgments ...................................................................................................................................... 2-8-10 Literature Cited .......................................................................................................................................... 2-8-10 2-8-2 Chapter 2-8: Anthocerotophyta CHAPTER 2-8 ANTHOCEROTOPHYTA Figure 1. Notothylas orbicularis thallus with involucres. Photo by Michael Lüth, with permission. Anthocerotophyta These plants, once placed among the bryophytes in the families. The second class is Leiosporocerotopsida, a Anthocerotae, now generally placed in the phylum class with one order, one family, and one genus. The genus Anthocerotophyta (hornworts, Figure 1), seem more Leiosporoceros differs from members of the class distantly related, and genetic evidence may even present -

Introduction to Common Native & Invasive Freshwater Plants in Alaska
Introduction to Common Native & Potential Invasive Freshwater Plants in Alaska Cover photographs by (top to bottom, left to right): Tara Chestnut/Hannah E. Anderson, Jamie Fenneman, Vanessa Morgan, Dana Visalli, Jamie Fenneman, Lynda K. Moore and Denny Lassuy. Introduction to Common Native & Potential Invasive Freshwater Plants in Alaska This document is based on An Aquatic Plant Identification Manual for Washington’s Freshwater Plants, which was modified with permission from the Washington State Department of Ecology, by the Center for Lakes and Reservoirs at Portland State University for Alaska Department of Fish and Game US Fish & Wildlife Service - Coastal Program US Fish & Wildlife Service - Aquatic Invasive Species Program December 2009 TABLE OF CONTENTS TABLE OF CONTENTS Acknowledgments ............................................................................ x Introduction Overview ............................................................................. xvi How to Use This Manual .................................................... xvi Categories of Special Interest Imperiled, Rare and Uncommon Aquatic Species ..................... xx Indigenous Peoples Use of Aquatic Plants .............................. xxi Invasive Aquatic Plants Impacts ................................................................................. xxi Vectors ................................................................................. xxii Prevention Tips .................................................... xxii Early Detection and Reporting -

The Supramolecular Organization of Self-Assembling Chlorosomal Bacteriochlorophyll C, D,Ore Mimics
The supramolecular organization of self-assembling chlorosomal bacteriochlorophyll c, d,ore mimics Tobias Jochum*†, Chilla Malla Reddy†‡, Andreas Eichho¨ fer‡, Gernot Buth*, Je¸drzej Szmytkowski§¶, Heinz Kalt§¶, David Moss*, and Teodor Silviu Balaban‡¶ʈ *Institute for Synchrotron Radiation, Karlsruhe Institute of Technology, Forschungszentrum Karlsruhe, Postfach 3640, D-76021 Karlsruhe, Germany; ‡Institute for Nanotechnology, Karlsruhe Institute of Technology, Forschungszentrum Karlsruhe, Postfach 3640, D-76021 Karlsruhe, Germany; §Institute of Applied Physics, Karlsruhe Institute of Technology, Universita¨t Karlsruhe (TH), D-76131 Karslruhe, Germany; and ¶Center for Functional Nanostructures, Universita¨t Karlsruhe (TH), D-76131 Karslruhe, Germany Edited by James R. Norris, University of Chicago, Chicago, IL, and accepted by the Editorial Board July 18, 2008 (received for review March 22, 2008) Bacteriochlorophylls (BChls) c, d, and e are the main light-harvesting direct structural evidence from x-ray single crystal structures, the pigments of green photosynthetic bacteria that self-assemble into exact nature of BChl superstructures remain elusive and these nanostructures within the chlorosomes forming the most efficient have been controversially discussed in the literature (1–3, 7, antennas of photosynthetic organisms. All previous models of the 11–17). It is now generally accepted that the main feature of chlorosomal antennae, which are quite controversially discussed chlorosomes is the self-assembly of BChls and that these pig- because no single crystals could be grown so far from these or- ments are not bound by a rigid protein matrix as is the case of ganelles, involve a strong hydrogen-bonding interaction between the other, well characterized light-harvesting systems (1). Small- 31 hydroxyl group and the 131 carbonyl group. -

1307 Fungi Representing 1139 Infrageneric Taxa, 317 Genera and 66 Families ⇑ Jolanta Miadlikowska A, , Frank Kauff B,1, Filip Högnabba C, Jeffrey C
Molecular Phylogenetics and Evolution 79 (2014) 132–168 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev A multigene phylogenetic synthesis for the class Lecanoromycetes (Ascomycota): 1307 fungi representing 1139 infrageneric taxa, 317 genera and 66 families ⇑ Jolanta Miadlikowska a, , Frank Kauff b,1, Filip Högnabba c, Jeffrey C. Oliver d,2, Katalin Molnár a,3, Emily Fraker a,4, Ester Gaya a,5, Josef Hafellner e, Valérie Hofstetter a,6, Cécile Gueidan a,7, Mónica A.G. Otálora a,8, Brendan Hodkinson a,9, Martin Kukwa f, Robert Lücking g, Curtis Björk h, Harrie J.M. Sipman i, Ana Rosa Burgaz j, Arne Thell k, Alfredo Passo l, Leena Myllys c, Trevor Goward h, Samantha Fernández-Brime m, Geir Hestmark n, James Lendemer o, H. Thorsten Lumbsch g, Michaela Schmull p, Conrad L. Schoch q, Emmanuël Sérusiaux r, David R. Maddison s, A. Elizabeth Arnold t, François Lutzoni a,10, Soili Stenroos c,10 a Department of Biology, Duke University, Durham, NC 27708-0338, USA b FB Biologie, Molecular Phylogenetics, 13/276, TU Kaiserslautern, Postfach 3049, 67653 Kaiserslautern, Germany c Botanical Museum, Finnish Museum of Natural History, FI-00014 University of Helsinki, Finland d Department of Ecology and Evolutionary Biology, Yale University, 358 ESC, 21 Sachem Street, New Haven, CT 06511, USA e Institut für Botanik, Karl-Franzens-Universität, Holteigasse 6, A-8010 Graz, Austria f Department of Plant Taxonomy and Nature Conservation, University of Gdan´sk, ul. Wita Stwosza 59, 80-308 Gdan´sk, Poland g Science and Education, The Field Museum, 1400 S. -

An Evolving Phylogenetically Based Taxonomy of Lichens and Allied Fungi
Opuscula Philolichenum, 11: 4-10. 2012. *pdf available online 3January2012 via (http://sweetgum.nybg.org/philolichenum/) An evolving phylogenetically based taxonomy of lichens and allied fungi 1 BRENDAN P. HODKINSON ABSTRACT. – A taxonomic scheme for lichens and allied fungi that synthesizes scientific knowledge from a variety of sources is presented. The system put forth here is intended both (1) to provide a skeletal outline of the lichens and allied fungi that can be used as a provisional filing and databasing scheme by lichen herbarium/data managers and (2) to announce the online presence of an official taxonomy that will define the scope of the newly formed International Committee for the Nomenclature of Lichens and Allied Fungi (ICNLAF). The online version of the taxonomy presented here will continue to evolve along with our understanding of the organisms. Additionally, the subfamily Fissurinoideae Rivas Plata, Lücking and Lumbsch is elevated to the rank of family as Fissurinaceae. KEYWORDS. – higher-level taxonomy, lichen-forming fungi, lichenized fungi, phylogeny INTRODUCTION Traditionally, lichen herbaria have been arranged alphabetically, a scheme that stands in stark contrast to the phylogenetic scheme used by nearly all vascular plant herbaria. The justification typically given for this practice is that lichen taxonomy is too unstable to establish a reasonable system of classification. However, recent leaps forward in our understanding of the higher-level classification of fungi, driven primarily by the NSF-funded Assembling the Fungal Tree of Life (AFToL) project (Lutzoni et al. 2004), have caused the taxonomy of lichen-forming and allied fungi to increase significantly in stability. This is especially true within the class Lecanoromycetes, the main group of lichen-forming fungi (Miadlikowska et al. -

The Macroevolutionary Dynamics of Symbiotic and Phenotypic Diversification in Lichens
The macroevolutionary dynamics of symbiotic and phenotypic diversification in lichens Matthew P. Nelsena,1, Robert Lückingb, C. Kevin Boycec, H. Thorsten Lumbscha, and Richard H. Reea aDepartment of Science and Education, Negaunee Integrative Research Center, The Field Museum, Chicago, IL 60605; bBotanischer Garten und Botanisches Museum, Freie Universität Berlin, 14195 Berlin, Germany; and cDepartment of Geological Sciences, Stanford University, Stanford, CA 94305 Edited by Joan E. Strassmann, Washington University in St. Louis, St. Louis, MO, and approved July 14, 2020 (received for review February 6, 2020) Symbioses are evolutionarily pervasive and play fundamental roles macroevolutionary consequences of ant–plant interactions (15–19). in structuring ecosystems, yet our understanding of their macroevo- However, insufficient attention has been paid to one of the most lutionary origins, persistence, and consequences is incomplete. We iconic examples of symbiosis (20, 21): Lichens. traced the macroevolutionary history of symbiotic and phenotypic Lichens are stable associations between a mycobiont (fungus) diversification in an iconic symbiosis, lichens. By inferring the most and photobiont (eukaryotic alga or cyanobacterium). The pho- comprehensive time-scaled phylogeny of lichen-forming fungi (LFF) tobiont supplies the heterotrophic fungus with photosynthetically to date (over 3,300 species), we identified shifts among symbiont derived carbohydrates, while the mycobiont provides the pho- classes that broadly coincided with the convergent -
Lichens of Alaska's South Coast
United States Department of Agriculture Lichens of Alaska’s South Coast Forest Service R10-RG-190 Alaska Region Reprint April 2014 WHAT IS A LICHEN? Lichens are specialized fungi that “farm” algae as a food source. Unlike molds, mildews, and mushrooms that parasitize or scavenge food from other organisms, the fungus of a lichen cultivates tiny algae and / or blue-green bacteria (called cyanobacteria) within the fabric of interwoven fungal threads that form the body of the lichen (or thallus). The algae and cyanobacteria produce food for themselves and for the fungus by converting carbon dioxide and water into sugars using the sun’s energy (photosynthesis). Thus, a lichen is a combination of two or sometimes three organisms living together. Perhaps the most important contribution of the fungus is to provide a protective habitat for the algae or cyanobacteria. The green or blue-green photosynthetic layer is often visible between two white fungal layers if a piece of lichen thallus is torn off. Most lichen-forming fungi cannot exist without the photosynthetic partner because they have become dependent on them for survival. But in all cases, a fungus looks quite different in the lichenized form compared to its free-living form. HOW DO LICHENS REPRODUCE? Lichens sexually reproduce with fruiting bodies of various shapes and colors that can often look like miniature mushrooms. These are called apothecia (Fig. 1) and contain spores that germinate and Figure 1. Apothecia, fruiting grow into the fungus. Each bodies fungus must find the right photosynthetic partner in order to become a lichen. Lichens reproduce asexually in several ways.