Attachment Bonds Moderate the Function of Ovulatory Cycle Adaptations
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Development of a Partner Preference Test That Differentiates Between Established Pair Bonds and Other Relationships in Socially
American Journal of Primatology RESEARCH ARTICLE Development of a Partner Preference Test that Differentiates Between Established Pair Bonds and Other Relationships in Socially Monogamous Titi Monkeys (Callicebus cupreus) SARAH B. CARP1#, EMILY S. ROTHWELL1,2*,#, ALEXIS BOURDON3, SARA M. FREEMAN1, EMILIO FERRER4, 1,2,4 AND KAREN L. BALES 1California National Primate Research Center, University of California—Davis, Davis, California 2Animal Behavior Graduate Group, University of California—Davis, Davis, California 3AgroParisTech, Paris, France 4Department of Psychology, University of California—Davis, Davis, California Partner preference, or the selective social preference for a pair mate, is a key behavioral indicator of social monogamy. Standardized partner preference testing has been used extensively in rodents but a single test has not been standardized for primates. The goal of this study was to develop a partner preference test with socially monogamous titi monkeys (Callicebus cupreus) adapted from the widely used rodent test. In Experiment 1, we evaluated the test with pairs of titi monkeys (N ¼ 12) in a three-chambered apparatus for 3 hr. The subject was placed in the middle chamber, with grated windows separating it from its partner on one side and an opposite sex stranger on the other side. Subjects spent a greater proportion of time in proximity to their partners’ windows than the strangers’, indicating a consistent preference for the partner over the stranger. Touching either window did not differ between partners and strangers, suggesting it was not a reliable measure of partner preference. Subjects chose their partner more than the stranger during catch and release sessions at the end of the test. -

Pair-Bonding, Romantic Love and Evolution: the Curious Case Of
PPSXXX10.1177/1745691614561683Fletcher et al.Pair-Bonding, Romantic Love, and Evolution 561683research-article2014 Perspectives on Psychological Science 2015, Vol. 10(1) 20 –36 Pair-Bonding, Romantic Love, and © The Author(s) 2014 Reprints and permissions: sagepub.com/journalsPermissions.nav Evolution: The Curious Case of DOI: 10.1177/1745691614561683 Homo sapiens pps.sagepub.com Garth J. O. Fletcher1, Jeffry A. Simpson2, Lorne Campbell3, and Nickola C. Overall4 1Victoria University Wellington, New Zealand; 2University of Minnesota; 3University of Western Ontario, Canada; and 4University of Auckland, New Zealand Abstract This article evaluates a thesis containing three interconnected propositions. First, romantic love is a “commitment device” for motivating pair-bonding in humans. Second, pair-bonding facilitated the idiosyncratic life history of hominins, helping to provide the massive investment required to rear children. Third, managing long-term pair bonds (along with family relationships) facilitated the evolution of social intelligence and cooperative skills. We evaluate this thesis by integrating evidence from a broad range of scientific disciplines. First, consistent with the claim that romantic love is an evolved commitment device, our review suggests that it is universal; suppresses mate-search mechanisms; has specific behavioral, hormonal, and neuropsychological signatures; and is linked to better health and survival. Second, we consider challenges to this thesis posed by the existence of arranged marriage, polygyny, divorce, and infidelity. Third, we show how the intimate relationship mind seems to be built to regulate and monitor relationships. Fourth, we review comparative evidence concerning links among mating systems, reproductive biology, and brain size. Finally, we discuss evidence regarding the evolutionary timing of shifts to pair-bonding in hominins. -

The Origins of the Institutions of Marriage
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Research Papers in Economics Q ED Queen’s Economics Department Working Paper No. 1180 The Origins of the Institutions of Marriage Marina E. Adshade Brooks A. Kaiser Dalhousie University Department of Economics Queen’s University 94 University Avenue Kingston, Ontario, Canada K7L 3N6 8-2008 THE ORIGIN OF THE INSTITUTIONS OF MARRIAGE Marina E. Adshade Brooks A. Kaiser August 13, 2008 Abstract Standard economic theories of household formation predict the rise of institutionalized polyg- yny in response to increased resource inequality among men. We propose a theory, within the framework of a matching model of marriage, in which, in some cases, institutionalized monogamy prevails, even when resources are unequally distributed, as a result of agricultural externalities that increase the presence of pair-bonding hormones. Within marriage, hormone levels contribute to the formation of the marital pair bond, the strength of which determines a man’swillingness to invest in his wife’schildren. These pair bonds are reinforced through phys- ical contact between the man and his wife and can be ampli…ed by externalities produced by certain production technologies. Both the presence of additional wives and the absence of these externalities reduce the strength of the marital bond and, where the …tness of a child is increas- ing in paternal investment, reduce a woman’sexpected lifetime fertility. Multiple equilibria in terms of the dominant form of marriage (for example, polygyny or monogamy) are possible, if the surplus to a match is a function of reproductive success as well as material income. -

Pair-Bonded Relationships and Romantic Alternatives 1
Pair-bonded Relationships and Romantic Alternatives 1 Running Head: PAIRBONDED RELATIONSHIPS AND ROMANTIC ALTERNATIVES Pair-bonded Relationships and Romantic Alternatives: Toward an Integration of Evolutionary and Relationship Science Perspectives Kristina M. Durante Rutgers University Paul W. Eastwick University of Texas at Austin Eli J. Finkel Northwestern University Steven W. Gangestad University of New Mexico Jeffry A. Simpson University of Minnesota, Twin Cities Campus Note: All authors contributed equally and are listed in alphabetical order. In Press, Advances in Experimental Social Psychology Pair-bonded Relationships and Romantic Alternatives 2 Abstract Relationship researchers and evolutionary psychologists have been studying human mating for decades, but research inspired by these two perspectives often yields fundamentally different images of how people mate. Research in the relationship science tradition frequently emphasizes ways in which committed relationship partners are motivated to maintain their relationships (e.g., by cognitively derogating attractive alternatives), whereas research in the evolutionary tradition frequently emphasizes ways in which individuals are motivated to seek out their own reproductive interests at the expense of partners’ (e.g., by surreptitiously having sex with attractive alternatives). Rather than being incompatible, the frameworks that guide each perspective have different assumptions that can generate contrasting predictions and can lead researchers to study the same behavior in different ways. This paper, which represents the first major attempt to bring the two perspectives together in a cross-fertilization of ideas, provides a framework to understand contrasting effects and guide future research. This framework—the conflict-confluence model— characterizes evolutionary and relationship science perspectives as being arranged along a continuum reflecting the extent to which mating partners’ interests are misaligned versus aligned. -

Oxytocin, Vasopressin and Pair Bonding: Implications for Autism Elizabeth A
Phil. Trans. R. Soc. B (2006) 361, 2187–2198 doi:10.1098/rstb.2006.1939 Published online 6 November 2006 Oxytocin, vasopressin and pair bonding: implications for autism Elizabeth A. D. Hammock and Larry J. Young* Department of Psychiatry and Behavioural Sciences, Centre for Behavioural Neuroscience, Yerkes National Primate Research Centre, Emory University, Atlanta, GA 30329, USA Understanding the neurobiological substrates regulating normal social behaviours may provide valuable insights in human behaviour, including developmental disorders such as autism that are characterized by pervasive deficits in social behaviour. Here, we review the literature which suggests that the neuropeptides oxytocin and vasopressin play critical roles in modulating social behaviours, with a focus on their role in the regulation of social bonding in monogamous rodents. Oxytocin and vasopressin contribute to a wide variety of social behaviours, including social recognition, communication, parental care, territorial aggression and social bonding. The effects of these two neuropeptides are species-specific and depend on species-specific receptor distributions in the brain. Comparative studies in voles with divergent social structures have revealed some of the neural and genetic mechanisms of social-bonding behaviour. Prairie voles are socially monogamous; males and females form long-term pair bonds, establish a nest site and rear their offspring together. In contrast, montane and meadow voles do not form a bond with a mate and only the females take part in rearing the young. Species differences in the density of receptors for oxytocin and vasopressin in ventral forebrain reward circuitry differentially reinforce social-bonding behaviour in the two species. High levels of oxytocin receptor (OTR) in the nucleus accumbens and high levels of vasopressin 1a receptor (V1aR) in the ventral pallidum contribute to monogamous social structure in the prairie vole. -

The Psychology of the Pair-Bond: Past and Future Contributions of Close Relationships Research to Evolutionary Psychology Paul W
This article was downloaded by: [Paul W. Eastwick] On: 02 September 2013, At: 10:01 Publisher: Routledge Informa Ltd Registered in England and Wales Registered Number: 1072954 Registered office: Mortimer House, 37-41 Mortimer Street, London W1T 3JH, UK Psychological Inquiry: An International Journal for the Advancement of Psychological Theory Publication details, including instructions for authors and subscription information: http://www.tandfonline.com/loi/hpli20 The Psychology of the Pair-Bond: Past and Future Contributions of Close Relationships Research to Evolutionary Psychology Paul W. Eastwick a a Department of Human Development and Family Sciences , The University of Texas at Austin , Austin , Texas Published online: 02 Sep 2013. To cite this article: Paul W. Eastwick (2013) The Psychology of the Pair-Bond: Past and Future Contributions of Close Relationships Research to Evolutionary Psychology, Psychological Inquiry: An International Journal for the Advancement of Psychological Theory, 24:3, 183-191 To link to this article: http://dx.doi.org/10.1080/1047840X.2013.816927 PLEASE SCROLL DOWN FOR ARTICLE Taylor & Francis makes every effort to ensure the accuracy of all the information (the “Content”) contained in the publications on our platform. However, Taylor & Francis, our agents, and our licensors make no representations or warranties whatsoever as to the accuracy, completeness, or suitability for any purpose of the Content. Any opinions and views expressed in this publication are the opinions and views of the authors, and are not the views of or endorsed by Taylor & Francis. The accuracy of the Content should not be relied upon and should be independently verified with primary sources of information. -

Intimate Relationships Then and Now: How Old Hormonal Processes Are Influenced by Our Modern Psychology
Adaptive Human Behavior and Physiology DOI 10.1007/s40750-015-0021-9 ORIGINAL ARTICLE Intimate Relationships Then and Now: How Old Hormonal Processes are Influenced by Our Modern Psychology Britney M. Wardecker & Leigh K. Smith & Robin S. Edelstein & Timothy J. Loving Received: 5 September 2014 /Revised: 13 January 2015 /Accepted: 20 January 2015 # Springer International Publishing 2015 Abstract In this review we argue that relatively recent evolutionary adaptations that are relational or psychological in nature might refocus, dampen, or otherwise shape hormonal processes related to evolutionarily Bolder^ behaviors. We focus on the steroid hormones testosterone and estradiol and discuss a) their associations with Bolder relational processes^ such as mate competition, dominance and nurturance, and b) the ways in which Bnewer relational processes^ such as commitment and attachment relate to these hormones in the context of intimate relationships. We propose that these new relational processes might influence hormones in a manner that enables short-term mating relationships to be transformed into long-term romantic pair-bonds that may be unique to humans. Keywords Mating . Pair-bonds . Testosterone . Estradiol . Evolution . Adaptations Most species, including humans, have evolved some repertoire of behaviors that facilitate short-term mating (i.e., uncommitted sexual relationships that last for a short period of time, Simpson and Gangestad 1991), such as attracting desirable mates, competing for the attention of those mates, and keeping others away from mates once they have been acquired (Gangestad and Simpson 2000). These evolutionarily Bold^ behaviors occur frequently, can be engaged with limited conscious effort (Miller 1998), and are important components of the relationship initiation process. -

Neuroendocrine Control in Social Relationships in Non-Human Primates: Field Based Evidence
YHBEH-04189; No. of pages: 15; 4C: Hormones and Behavior xxx (2017) xxx–xxx Contents lists available at ScienceDirect Hormones and Behavior journal homepage: www.elsevier.com/locate/yhbeh Review article Neuroendocrine control in social relationships in non-human primates: Field based evidence Toni E. Ziegler a,⁎, Catherine Crockford b a Wisconsin National Primate Research Center, University of Wisconsin, Madison, WI, USA b Max Plank Institute for Evolutionary Anthropology, Leipzig, Germany article info abstract Article history: Primates maintain a variety of social relationships and these can have fitness consequences. Research has Received 1 March 2016 established that different types of social relationships are unpinned by different or interacting hormonal systems, Revised 6 March 2017 for example, the neuropeptide oxytocin influences social bonding, the steroid hormone testosterone influences Accepted 7 March 2017 dominance relationships, and paternal care is characterized by high oxytocin and low testosterone. Although Available online xxxx the oxytocinergic system influences social bonding, it can support different types of social bonds in different spe- Keywords: cies, whether pair bonds, parent-offspring bonds or friendships. It seems that selection processes shape social and Oxytocin mating systems and their interactions with neuroendocrine pathways. Within species, there are individual differ- Prolactin ences in the development of the neuroendocrine system: the social environment individuals are exposed to dur- Tend-and-defend ing ontogeny alters their neuroendocrine and socio-cognitive development, and later, their social interactions as Social bonds adults. Within individuals, neuroendocrine systems can also have short-term effects, impacting on social interac- In-group/out-group tions, such as those during hunting, intergroup encounters or food sharing, or the likelihood of cooperating, win- Cooperation ning or losing. -

Pair Bonding: What Mediates Its Formation and Maintenance?
Pair bonding: what mediates its formation and maintenance? by Amanda M. Dios A Dissertation submitted to Graduate School-Newark Rutgers, The State University of New Jersey for the degree of Doctor of Philosophy in Psychology written under the direction of Mei-Fang Cheng and approved by ___________________________________ Mei-Fang Cheng ___________________________________ Michael Shifflet ___________________________________ Michael Stephen J. Hanson ___________________________________Shifflet Mauricio Delgado ___________________________________ Mark Hauber Newark, NJ May 2015 Copyright page: © [2015] Amanda Dios ALL RIGHTS RESERVED ABSTRACT OF THE DISSERTATION Pair bonding: what mediates its formation and maintenance? BY: AMANDA DIOS Dissertation Director: Mei Fang Cheng Pair bonding is an exclusive mating relationship associating the memory of a mate with the potential successful completion of a breeding cycle. In evolutionary biology, pair bonding has been studied as a mating strategy and this biological phenomenon has been associated with survival related reproductive behaviors, however, behaviors that function in the formation of bonding and those that are established as a result of bonding are seldom distinguished, making it difficult to assess how pair bonding is represented in the brain. This thesis assesses pair bonding in ring doves, an animal that has a predictable sequence leading to a successful breeding cycle. Through a series of lesion, immunohistochemistry, and behavior studies we sought to understand the processing and execution of this fitness-critical complex behavior, by assessing the neural basis of pair bonding, understanding which behavioral events initiate the formation of the bond and how bonding affects an animal's decisions involving a mate. We found a neuro- marker for pair bonding that is more accurate than current methods of measuring bonding. -

Oxytocin and Romantic Relationships: a Functional Perspective Nicholas Grebe
University of New Mexico UNM Digital Repository Psychology ETDs Electronic Theses and Dissertations 9-1-2015 Oxytocin and Romantic Relationships: A Functional Perspective Nicholas Grebe Follow this and additional works at: https://digitalrepository.unm.edu/psy_etds Recommended Citation Grebe, Nicholas. "Oxytocin and Romantic Relationships: A Functional Perspective." (2015). https://digitalrepository.unm.edu/ psy_etds/52 This Thesis is brought to you for free and open access by the Electronic Theses and Dissertations at UNM Digital Repository. It has been accepted for inclusion in Psychology ETDs by an authorized administrator of UNM Digital Repository. For more information, please contact [email protected]. i Nicholas M. Grebe Candidate Psychology Department This thesis is approved, and it is acceptable in quality and form for publication: Approved by the Thesis Committee: Steven W. Gangestad , Chairperson Melissa Emery Thompson Marco Del Giudice ii OXYTOCIN AND ROMANTIC RELATIONSHIPS: A FUNCTIONAL PERSPECTIVE BY NICHOLAS M. GREBE BACHELOR OF ARTS, PSYCHOLOGY UNIVERSITY OF COLORADO, MAY 2009 THESIS Submitted in Partial Fulfillment of the Requirements for the Degree of Master of Science Psychology The University of New Mexico Albuquerque, New Mexico July 2015 iii ACKNOWLEDGMENTS There are many, many people who have aided me, directly or indirectly, with the completion of this step in my academic career. I will try to just name a few. First, thanks to my advisor, Dr. Steve Gangestad. I am so very fortunate to have a mentor that has all the qualities one could want: brilliant, well-respected by his colleagues and anyone who knows him, patient with his time, and an endless source of good advice. -
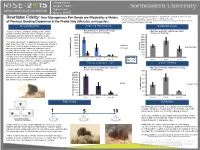
How Monogamous Pair Bond Are Affected by a History of Previous Bonding Experience in the Prairie Vole
Undergraduate Category: Health Degree Level: Abstract ID# 872 Tedi Rosenstein1, Naomi Zingman-Daniels1, Amy Kristl1, Craig F. Ferris1,3, C. Sue Rewritable Fidelity: How Monogamous Pair Bonds are Affected by a History Carter1,2, Allison Perkeybile1, Jason R. Yee1, William Kenkel1 1 Northeastern University, Dept. of Psychology, Center for Translational Neuroimaging; 2The Kinsey Institute, of Previous Bonding Experience in the Prairie Vole (Microtus ochrogaster) Indiana University; 3Ekam Imaging, Boston MA BACKGROUND PARTNER PREFERENCE SOLITARY CAGE Male preference for partner and stranger Social monogamy is a fundamental aspect of the human Male time spent in the solitary cage did not females declines over 10 pairings. condition whereby males and females undergo attachment. change over 10 pairings. 4000 4500 Reproduction and social behavior are based on a flexible physiological foundation which can accommodate life experience. 3500 4000 3000 3500 The prairie vole provides an optimal model of social monogamy 2500 3000 within which it is possible to examine the biological underpinning of 2500 attachment. Social monogamy is defined as a mating strategy in 2000 Partner 2000 Solitary Cage which one breeding female and one breeding male closely 1500 Stranger Time (sec) 1500 associate with one another over several breeding seasons. It is 1000 hypothesized that social monogamy evolved due to the male’s 1000 inability to defend and monopolize multiple females. Socially 500 500 monogamous behavior is physiologically regulated by (sec) Huddling Time spent 0 Sample Sizes: 0 neuropeptides, such as oxytocin and vasopressin, as well as other Pair 1 n = 24 Pair 1 Pair 5 Pair 10 Pair 1 Pair 5 Pair 10 biological mechanisms. -

The Neurobiology of Pair Bond Formation, Bond Disruption, and Social Buffering
Available online at www.sciencedirect.com ScienceDirect The neurobiology of pair bond formation, bond disruption, and social buffering 1 2 Claudia Lieberwirth and Zuoxin Wang Abstract and the similarity of neural mechanisms across monoga- mous species, for example, [8–10]. Consequently, these Enduring social bonds play an essential role in human society. observations spurred the interest to develop appropriate These bonds positively affect psychological, physiological, and animal models to study the neurobiological mechanisms behavioral functions. Here, we review the recent literature on underlying pair bonding. the neurobiology, particularly the role of oxytocin and dopamine, of pair bond formation, bond disruption, and social As pair bond formation and the associated behaviors (e.g., buffering effects on stress responses, from studies utilizing the mate guarding and bi-parental care) are only displayed by socially monogamous prairie vole (Microtus ochrogaster). 3–5% of mammals [11,12], there are very few appropriate Addresses 1 animal models. Here we will focus on the prairie vole Behavioral Science Department, Utah Valley University, Orem, UT 84057, USA (Microtus ochrogaster) that has been used to study the 2 Department of Psychology and Program in Neuroscience, Florida State neurobiological mechanisms of pair bonding [13]. We University, Tallahassee, FL 32306, USA will discuss the role of the neurochemicals oxytocin (OT) and dopamine (DA) in pair bond formation. Then, Corresponding author: Lieberwirth, Claudia ([email protected]) we will discuss some recent findings highlighting the importance of OT and DA during bond disruption and Current Opinion in Neurobiology 2016, 40:8–13 in mediating social buffering effects on stress responses. This review comes from a themed issue on Systems neuroscience Edited by Don Katz and Leslie Kay The prairie vole model The prairie vole is a socially monogamous rodent species.