Mechanics and Microstructure of the Atrioventricular Heart Valve Chordae Tendineae: a Review
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Distance Learning Program Anatomy of the Human Heart/Pig Heart Dissection Middle School/ High School
Distance Learning Program Anatomy of the Human Heart/Pig Heart Dissection Middle School/ High School This guide is for middle and high school students participating in AIMS Anatomy of the Human Heart and Pig Heart Dissections. Programs will be presented by an AIMS Anatomy Specialist. In this activity students will become more familiar with the anatomical structures of the human heart by observing, studying, and examining human specimens. The primary focus is on the anatomy and flow of blood through the heart. Those students participating in Pig Heart Dissections will have the opportunity to dissect and compare anatomical structures. At the end of this document, you will find anatomical diagrams, vocabulary review, and pre/post tests for your students. National Science Education (NSES) Content Standards for grades 9-12 • Content Standard:K-12 Unifying Concepts and Processes :Systems order and organization; Evidence, models and explanation; Form and function • Content Standard F, Science in Personal and Social Perspectives: Personal and community health • Content Standard C, Life Science: Matter, energy and organization of living systems • Content Standard A Science as Inquiry National Science Education (NSES) Content Standards for grades 5-8 • Content Standard A Science as Inquiry • Content Standard C, Life Science: Structure and function in living systems; Diversity and adaptations of organisms • Content Standard F, Science in Personal and Social Perspectives: Personal Health Show Me Standards (Science and Health/Physical Education) • Science 3. Characteristics and interactions of living organisms • Health/Physical Education 1. Structures of, functions of and relationships among human body systems Objectives: The student will be able to: 1. -

Prep for Practical II
Images for Practical II BSC 2086L "Endocrine" A A B C A. Hypothalamus B. Pineal Gland (Body) C. Pituitary Gland "Endocrine" 1.Thyroid 2.Adrenal Gland 3.Pancreas "The Pancreas" "The Adrenal Glands" "The Ovary" "The Testes" Erythrocyte Neutrophil Eosinophil Basophil Lymphocyte Monocyte Platelet Figure 29-3 Photomicrograph of a human blood smear stained with Wright’s stain (765). Eosinophil Lymphocyte Monocyte Platelets Neutrophils Erythrocytes "Blood Typing" "Heart Coronal" 1.Right Atrium 3 4 2.Superior Vena Cava 5 2 3.Aortic Arch 6 4.Pulmonary Trunk 1 5.Left Atrium 12 9 6.Bicuspid Valve 10 7.Interventricular Septum 11 8.Apex of The Heart 9. Chordae tendineae 10.Papillary Muscle 7 11.Tricuspid Valve 12. Fossa Ovalis "Heart Coronal Section" Coronal Section of the Heart to show valves 1. Bicuspid 2. Pulmonary Semilunar 3. Tricuspid 4. Aortic Semilunar 5. Left Ventricle 6. Right Ventricle "Heart Coronal" 1.Pulmonary trunk 2.Right Atrium 3.Tricuspid Valve 4.Pulmonary Semilunar Valve 5.Myocardium 6.Interventricular Septum 7.Trabeculae Carneae 8.Papillary Muscle 9.Chordae Tendineae 10.Bicuspid Valve "Heart Anterior" 1. Brachiocephalic Artery 2. Left Common Carotid Artery 3. Ligamentum Arteriosum 4. Left Coronary Artery 5. Circumflex Artery 6. Great Cardiac Vein 7. Myocardium 8. Apex of The Heart 9. Pericardium (Visceral) 10. Right Coronary Artery 11. Auricle of Right Atrium 12. Pulmonary Trunk 13. Superior Vena Cava 14. Aortic Arch 15. Brachiocephalic vein "Heart Posterolateral" 1. Left Brachiocephalic vein 2. Right Brachiocephalic vein 3. Brachiocephalic Artery 4. Left Common Carotid Artery 5. Left Subclavian Artery 6. Aortic Arch 7. -

Chapter 12 the Cardiovascular System: the Heart Pages
CHAPTER 12 THE CARDIOVASCULAR SYSTEM: THE HEART PAGES 388 - 411 LOCATION & GENERAL FEATURES OF THE HEART TWO CIRCUIT CIRCULATORY SYSTEM DIVISIONS OF THE HEART FOUR CHAMBERS Right Atrium Left Atrium Receives blood from Receives blood from the systemic circuit the pulmonary circuit FOUR CHAMBERS Right Ventricle Left Ventricle Ejects blood into the Ejects blood into the pulmonary circuit systemic circuit FOUR VALVES –ATRIOVENTRICULAR VALVES Right Atrioventricular Left Atrioventricular Valve (AV) Valve (AV) Tricuspid Valve Bicuspid Valve and Mitral Valve FOUR VALVES –SEMILUNAR VALVES Pulmonary valve Aortic Valve Guards entrance to Guards entrance to the pulmonary trunk the aorta FLOW OF BLOOD MAJOR VEINS AND ARTERIES AROUND THE HEART • Arteries carry blood AWAY from the heart • Veins allow blood to VISIT the heart MAJOR VEINS AND ARTERIES ON THE HEART Coronary Circulation – Supplies blood to the muscle tissue of the heart ARTERIES Elastic artery: Large, resilient vessels. pulmonary trunk and aorta Muscular artery: Medium-sized arteries. They distribute blood to skeletal muscles and internal organs. external carotid artery of the neck Arteriole: Smallest of arteries. Lead into capillaries VEINS Large veins: Largest of the veins. Superior and Inferior Vena Cava Medium-sized veins: Medium sized veins. Pulmonary veins Venules: the smallest type of vein. Lead into capillaries CAPILLARIES Exchange of molecules between blood and interstitial fluid. FLOW OF BLOOD THROUGH HEART TISSUES OF THE HEART THE HEART WALL Pericardium Outermost layer Serous membrane Myocardium Middle layer Thick muscle layer Endocardium Inner lining of pumping chambers Continuous with endothelium CARDIAC MUSCLE Depend on oxygen to obtain energy Abundant in mitochondria In contact with several other cardiac muscles Intercalated disks – interlocking membranes of adjacent cells Desmosomes Gap junctions CONNECTIVE TISSUE Wrap around each cardiac muscle cell and tie together adjacent cells. -
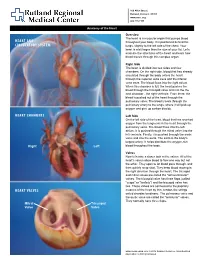
Heart and Circulatory System Heart Chambers
160 Allen Street Rutland, Vermont 05701 www.rrmc.org 802.775.7111 Anatomy of the Heart Overview The heart is a muscular organ that pumps blood HEART AND throughout your body. It is positioned behind the CIRCULATORY SYSTEM lungs, slightly to the left side of the chest. Your heart is a bit larger than the size of your fist. Let's examine the structures of the heart and learn how blood travels through this complex organ. Right Side The heart is divided into two sides and four chambers. On the right side, blood that has already circulated through the body enters the heart through the superior vena cava and the inferior vena cava. The blood flows into the right atrium. When this chamber is full, the heart pushes the blood through the tricuspid valve and into the the next chamber - the right ventricle. From there, the blood is pushed out of the heart through the pulmonary valve. The blood travels through the pulmonary artery to the lungs, where it will pick up oxygen and give up carbon dioxide. HEART CHAMBERS Left Side On the left side of the heart, blood that has received oxygen from the lungs enters the heart through the pulmonary veins. The blood flows into the left atrium. It is pushed through the mitral valve into the left ventricle. Finally, it is pushed through the aortic valve and into the aorta. The aorta is the body's largest artery. It helps distribute the oxygen-rich Right Left blood throughout the body. Valves Now let's take a closer look at the valves. -

PHV Short.Indd
Anatomy & physiology: The heart Cardiac electrical conductance A Sinoatrial (SA) node D Right bundle branch B Atrioventricular (AV) node E Left bundle branch A C Atrioventricular bundle of His F Purkinje fibres The sinoatrial (SA) node is the heart's natural pacemaker, B containing cells that generate electrical impulses which spread F through the atria, triggering contraction of the atria, forcing blood into the ventricles, and stimulating the atrioventricular A (AV) node. E The AV node is linked with the atrioventricular bundle of His C which transmits impulses to the left and right bundle branches and then to the Purkinje fibres which surround the ventricles. The electrical impulses travel through the Purkinje fibres D causing the ventricles to contract and blood forced out of the heart. F Electrocardiogram (ECG) Heart construction The heart is a muscular, cone-shaped organ located behind The ECG traces the course of the cardiac impulse by the sternum and is approximately the same size as the recording the change in electrical potential on the surface of patient's closed fist. the body. Various parts of the ECG are associated with the travel of electrical impulses though the heart. The heart walls are made up the three structures: the pericardium, myocardium and endocardium. The P wave represents atrial depolarisation which causes the atria to contract. The pericardium is a thick fibrous membrane which surrounds the heart. Its function is to anchor the heart and Q is when the impulses arrive at the atrioventricular (AV) prevents over distension (expanding too much). node. The myocardium is the central layer of the heart and is The QRS complex represents ventricular depolarisation and formed from cardiac muscle tissue, it is this muscle which atrial repolarisation (ventricles contract and atria relax and provides the force which pumps the blood around the body. -

Sheep Heart Dissection
Sheep Heart Dissection Sheep heart (anterior view) This image shows an external view of the anterior side of a preserved sheep heart. Note the pointed apex of the heart and the wide superior end of the heart which is termed the base. The large blood vessels (i.e., the great vessels of the heart) which carry blood to and from the heart are located at the base. The right and left atria are also located at the base and appear as thin-walled chambers with ir- regular, more or less scalloped edges. The wrinkled portion of each atrium that protrudes externally to form a pouch is called the auricle or atrial appendage. The atria serve as receiving chambers for low pressure venous blood returning to the heart thus their walls are extremely thin. Observe the anterior interventricular sulcus extending from the left side of the base obliquely to the heart’s right side. The interventricular sulcus contains the left anterior descending coronary artery and the left coronary vein embedded within adipose tissue. The right ventricle lies to your left and toward the base relative to the anterior interventricular sulcus. The left ventricle lies to the right of the anterior interventricular sulcus and extends to and includes the apex of the heart. The ventricles are the pumping chambers of the heart and are, of necessity, thick walled. 1. Right ventricle - 2. Left ventricle - 3. Auricle of left atrium - 4. Pulmonary trunk 5. Aorta - 6. Interventricular sulcus Sheep Heart (posterior view) This image shows an external view of a preserved sheep heart. -

Congenital Aortic Valve Disease with Rupture of Mitral Chordae Tendineae
Br Heart J: first published as 10.1136/hrt.38.7.665 on 1 July 1976. Downloaded from British Heart Journal, 1976, 38, 665-673. Congenital aortic valve disease with rupture of mitral chordae tendineae Simon Joseph, Richard Emanuel, Marvin Sturridge, and Eckhardt Olsen From The Middlesex Hospital; Cardiothoracic Institute; and The National Heart Hospital, London A new clinical entity is described in which free aortic regurgitation from congenital aortic valve disease caused rupture of the chordae to the anterior leaflet of the mitral valve in 7 men aged 45 to 63years (mean 52years); 2 of the patients also had rupture ofchordae to the posterior leaflet. Comparing these patients with those with ruptured mitral chordae in association with rheumatic heart disease and patients with spontaneous chordal rupture, differences were evident. No patient had a history of rheumatic fever and none had active infection. The typical clinical presentation was of acute mitral regurgitation into a small left atrium, with severe pulmonary oedema which was often resistant to medical treatment. The cause ofchordal rupture in these patients was in part the result ofprogressive left ventricular dilatation, of direct trauma to the anterior cusp of the mitral valve, and possibly of a geneticfactor. The anatomicalfeatures of both aortic and mitral valves are described, and in 3 histology of the mitral valve was available; 2 had myxomatous degeneration similar to that seen in patients with spontaneous chordal rupture, and in 1 there was degeneration of collagen tissue. All patients were treated surgically but the mortality was high (5 out of 7, 70%). Early operation with replacement of the aortic and mitral valves is recommended if this high mortality is to be reduced. -

22. Heart.Pdf
CARDIOVASCULAR SYSTEM OUTLINE 22.1 Overview of the Cardiovascular System 657 22.1a Pulmonary and Systemic Circulations 657 22.1b Position of the Heart 658 22 22.1c Characteristics of the Pericardium 659 22.2 Anatomy of the Heart 660 22.2a Heart Wall Structure 660 22.2b External Heart Anatomy 660 Heart 22.2c Internal Heart Anatomy: Chambers and Valves 660 22.3 Coronary Circulation 666 22.4 How the Heart Beats: Electrical Properties of Cardiac Tissue 668 22.4a Characteristics of Cardiac Muscle Tissue 668 22.4b Contraction of Heart Muscle 669 22.4c The Heart’s Conducting System 670 22.5 Innervation of the Heart 672 22.6 Tying It All Together: The Cardiac Cycle 673 22.6a Steps in the Cardiac Cycle 673 22.6b Summary of Blood Flow During the Cardiac Cycle 673 22.7 Aging and the Heart 677 22.8 Development of the Heart 677 MODULE 9: CARDIOVASCULAR SYSTEM mck78097_ch22_656-682.indd 656 2/14/11 4:29 PM Chapter Twenty-Two Heart 657 n chapter 21, we discovered the importance of blood and the which carry blood back to the heart. The differences between I myriad of substances it carries. To maintain homeostasis, blood these types of vessels are discussed in chapter 23. Most arteries must circulate continuously throughout the body. The continual carry blood high in oxygen (except for the pulmonary arteries, pumping action of the heart is essential for maintaining blood as explained later), while most veins carry blood low in oxygen circulation. If the heart fails to pump adequate volumes of blood, (except for the pulmonary veins). -

The Material Properties of the Chordae Tendineae of the Mitral Valve: an in Vitro Investigation
THE MATERIAL PROPERTIES OF THE CHORDAE TENDINEAE OF THE MITRAL VALVE: AN IN VITRO INVESTIGATION A Thesis Presented to The Academic Faculty By Jennifer Lynn Ritchie In Partial Fulfillment Of the Requirements for the Degree Master of Science in Bioengineering Georgia Institute of Technology August, 2004 THE MATERIAL PROPERTIES OF THE CHORDAE TENDINEAE OF THE MITRAL VALVE: AN IN VITRO INVESTIGATION Approved By: Dr. Ajit P. Yoganathan, Advisor Dr. Raymond Vito Dr. Robert Guldberg Dr. Michael Sacks Date Approved: August 19, 2004 For my family and husband, without whom I would not have survived. ACKNOWLEDGEMENTS First and foremost, I would like to thank my family for always being by my side. Without their continual support, I would not have survived. I would like to especially thank my husband Steve for being by my side the entire time, making all the trips to Atlanta to visit me, and not allowing me to give up. I love you more than you know. Special thanks goes to my advisor Dr. Ajit P. Yoganathan for his support and exceptional opportunities he gave me during my time at Georgia Tech. I would also like to thank Dr. Raymond Vito, Dr. Robert Guldberg, and Dr. Michael Sacks for being part of my thesis committee. I would like to thank all the members of the Cardiovascular Fluid Dynamics Laboratory for your support and friendship. Zhaoming and Jorge, thank you for everything you helped me to accomplish. Without you, I would not have been able to complete this work. Dennis, thank you for developing the autotracking software! James and Tracey, thanks for all your help with the histology and biochemical work. -

1. Right Coronary 2. Left Anterior Descending 3. Left
1. RIGHT CORONARY 2. LEFT ANTERIOR DESCENDING 3. LEFT CIRCUMFLEX 4. SUPERIOR VENA CAVA 5. INFERIOR VENA CAVA 6. AORTA 7. PULMONARY ARTERY 8. PULMONARY VEIN 9. RIGHT ATRIUM 10. RIGHT VENTRICLE 11. LEFT ATRIUM 12. LEFT VENTRICLE 13. PAPILLARY MUSCLES 14. CHORDAE TENDINEAE 15. TRICUSPID VALVE 16. MITRAL VALVE 17. PULMONARY VALVE Coronary Arteries Because the heart is composed primarily of cardiac muscle tissue that continuously contracts and relaxes, it must have a constant supply of oxygen and nutrients. The coronary arteries are the network of blood vessels that carry oxygen- and nutrient-rich blood to the cardiac muscle tissue. The blood leaving the left ventricle exits through the aorta, the body’s main artery. Two coronary arteries, referred to as the "left" and "right" coronary arteries, emerge from the beginning of the aorta, near the top of the heart. The initial segment of the left coronary artery is called the left main coronary. This blood vessel is approximately the width of a soda straw and is less than an inch long. It branches into two slightly smaller arteries: the left anterior descending coronary artery and the left circumflex coronary artery. The left anterior descending coronary artery is embedded in the surface of the front side of the heart. The left circumflex coronary artery circles around the left side of the heart and is embedded in the surface of the back of the heart. Just like branches on a tree, the coronary arteries branch into progressively smaller vessels. The larger vessels travel along the surface of the heart; however, the smaller branches penetrate the heart muscle. -

Anatomy and Physiology of the Cardiovascular System
Chapter © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC 5 NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION Anatomy© Jonesand & Physiology Bartlett Learning, LLC of © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION the Cardiovascular System © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION OUTLINE Aortic arch: The second section of the aorta; it branches into Introduction the brachiocephalic trunk, left common carotid artery, and The Heart left subclavian artery. Structures of the Heart Aortic valve: Located at the base of the aorta, the aortic Conduction System© Jones & Bartlett Learning, LLCvalve has three cusps and opens© Jonesto allow blood & Bartlett to leave the Learning, LLC Functions of the HeartNOT FOR SALE OR DISTRIBUTIONleft ventricle during contraction.NOT FOR SALE OR DISTRIBUTION The Blood Vessels and Circulation Arteries: Elastic vessels able to carry blood away from the Blood Vessels heart under high pressure. Blood Pressure Arterioles: Subdivisions of arteries; they are thinner and have Blood Circulation muscles that are innervated by the sympathetic nervous Summary© Jones & Bartlett Learning, LLC system. © Jones & Bartlett Learning, LLC Atria: The upper chambers of the heart; they receive blood CriticalNOT Thinking FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION Websites returning to the heart. Review Questions Atrioventricular node (AV node): A mass of specialized tissue located in the inferior interatrial septum beneath OBJECTIVES the endocardium; it provides the only normal conduction pathway between the atrial and ventricular syncytia. -

Severe Restrictive Aortic Regurgitation Resulting from Valve Tenting by Unusual Aortic Chordae Tendineae Strands
Bahrain Medical Bulletin, Vol. 41, No. 2, June 2019 Severe Restrictive Aortic Regurgitation Resulting from Valve Tenting by Unusual Aortic Chordae Tendineae Strands Mohammed Abouelela, MD* Angel Espinosa, MD** Nazar A. Bukamal, MD*** Nitin R. Patani, MD**** Habib Tareef, MD***** Dinesh Babu, MD****** Nezar A. Al Saeedi, MD******* A fifty-eight-year-old male presented with severe aortic regurgitation (AR) due to abnormal fibrous chordae tendineae bands. Successful aortic valve replacement was performed where the chordae tendineae were removed. Bahrain Med Bull 2019; 41(2): 119 - 120 A non-functioning aortic valve is caused either by valve ultrasound confirmed by topographic and functional images. It stenosis or regurgitation. Aortic valve regurgitation is called also revealed an impaired left ventricle systolic function with valve insufficiency; the valve leaflets do not close completely ejection fraction of about 35%. The echo showed also normal causing the blood to flow back to the heart instead of being fully tricuspid valve with no tricuspid regurgitation, see figure 1. ejected to the aorta. The clinical onset of aortic regurgitation (AR) is usually insidious and progresses slowly, except in cases of infective endocarditis (IE) or aortic dissection1. Aortic insufficiency could be due to prolapse of the valve leaflets, or a congenitally deformed valve, infected valve (endocarditis), or due to dilation of aorta (aortic aneurysm), holes in leaflets, rheumatic valve disease or in rare cases where the aortic valves are attached to unusual aortic chordae tendineae in our case. Similar cases were reported2,3. Figure 1 (A) Figure 1 (B) The aim of this presentation is to report a case of severe aortic regurgitation (AR) due to abnormal fibrous chordae tendineae bands which was successfully treated surgically.