Studies on New and Old World Leishmaniases and Their
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Public Sector Development Programme 2019-20 (Original)
GOVERNMENT OF BALOCHISTAN PLANNING & DEVELOPMENT DEPARTMENT PUBLIC SECTOR DEVELOPMENT PROGRAMME 2019-20 (ORIGINAL) Table of Contents S.No. Sector Page No. 1. Agriculture……………………………………………………………………… 2 2. Livestock………………………………………………………………………… 8 3. Forestry………………………………………………………………………….. 11 4. Fisheries…………………………………………………………………………. 13 5. Food……………………………………………………………………………….. 15 6. Population welfare………………………………………………………….. 16 7. Industries………………………………………………………………………... 18 8. Minerals………………………………………………………………………….. 21 9. Manpower………………………………………………………………………. 23 10. Sports……………………………………………………………………………… 25 11. Culture……………………………………………………………………………. 30 12. Tourism…………………………………………………………………………... 33 13. PP&H………………………………………………………………………………. 36 14. Communication………………………………………………………………. 46 15. Water……………………………………………………………………………… 86 16. Information Technology…………………………………………………... 105 17. Education. ………………………………………………………………………. 107 18. Health……………………………………………………………………………... 133 19. Public Health Engineering……………………………………………….. 144 20. Social Welfare…………………………………………………………………. 183 21. Environment…………………………………………………………………… 188 22. Local Government ………………………………………………………….. 189 23. Women Development……………………………………………………… 198 24. Urban Planning and Development……………………………………. 200 25. Power…………………………………………………………………………….. 206 26. Other Schemes………………………………………………………………… 212 27. List of Schemes to be reassessed for Socio-Economic Viability 2-32 PREFACE Agro-pastoral economy of Balochistan, periodically affected by spells of droughts, has shrunk livelihood opportunities. -

MBBS / BDS ADMISSIONS Government Medical Colleges of Azad Jammu & Kashmir (AJ&K) and Reserved Seats for AJ&K Nationals in Pakistan, Session 2019-2020
University of Health Sciences Lahore MBBS / BDS ADMISSIONS Government Medical Colleges of Azad Jammu & Kashmir (AJ&K) and Reserved Seats for AJ&K Nationals in Pakistan, Session 2019-2020 Online applications are invited from eligible (First Class State Subject) candidates for admissions in First Year MBBS and BDS against reserved seats for AJ&K Nationals, Refugees 1947and Refugees 1989 (conditions apply), in the following Public Sector Medical/Dental Colleges of Pakistan (Punjab, Khyber Pakhtunkhwa, Balochistan & Sindh) and Public Sector Medical Colleges of AJ&K. Admissions will be made strictly on merit basis as per PM&DC Admission Regulations and Admission Policy of AJ&K Government in vogue: Medical/Dental Institutions of Pakistan Punjab (MBBS) Khyber Pakhtunkhwa (MBBS) Allama Iqbal Medical University Lahore Ayub Medical College Abbottabad Fatima Jinnah Medical University Lahore Gomal Medical College D.I Khan King Edward Medical University Lahore Khyber Medical University Peshawar Nishtar Medical University Multan Saidu Sharif Medical College Swat Punjab Medical University Faisalabad Khyber Pakhtunkhwa (BDS) Quaid e Azam Medical College Bahawalpur Dental Unit Ayub Medical College Abbottabad Rawalpindi Medical University Rawalpindi Sindh (MBBS) Services Institute of Medical Sciences Lahore Chandka Medical College Larkana Sheikh Zayad Medical College Rahim Yar Khan Balochistan (MBBS) Punjab (BDS) Bolan Medical College Quetta de’Montmorency College of Dentistry Lahore Medical Institutions of AJ&K Azad Jammu Kashmir Medical College Muzaffarabad Mohtarma Be’Nazir Bhutto Shaheed Medical College Mirpur Poonch Medical College Rawalakot 1. ELIGIBILITY CRITERIA i) Qualifications: In accordance with “MBBS and BDS (Admissions, House Job and Internship) Regulations, 2018, as amended on 30th May, 2019” of Pakistan Medical and Dental Council, the required qualifications for admissions are as follows: The applicant has passed, obtaining minimum Seventy percent (770/1100) marks, in Higher Secondary School Certificate (HSSC) or F.Sc. -

Multilocus Enzyme Electrophoresis And
Am. J. Trop. Med. Hyg., 75(2), 2006, pp. 261–266 Copyright © 2006 by The American Society of Tropical Medicine and Hygiene MULTILOCUS ENZYME ELECTROPHORESIS AND CYTOCHROME B GENE SEQUENCING–BASED IDENTIFICATION OF LEISHMANIA ISOLATES FROM DIFFERENT FOCI OF CUTANEOUS LEISHMANIASIS IN PAKISTAN JORGE D. MARCO,* ABDUL M. BHUTTO, FAROOQ R. SOOMRO, JAVED H. BALOCH, PAOLA A. BARROSO, HIROTOMO KATO, HIROSHI UEZATO, KEN KATAKURA, MASATAKA KORENAGA, SHIGEO NONAKA, AND YOSHIHISA HASHIGUCHI Department of Parasitology, Kochi Medical School, Kochi University, Kochi, Japan; Instituto de Patología Experimental, Facultad de Ciencias de la Salud, Universidad Nacional de Salta/Consejo Nacional de Investigaciones Científicas y Técnicas, Salta, Argentina; Department of Dermatology and Incharge Leprosy Unit, Chandka Medical College/Hospital Larkana, Sindh, Pakistan; Department of Veterinary Hygiene, Faculty of Agriculture, Yamaguchi University, Yamaguchi, Japan; Department of Dermatology, Faculty of Medicine, University of the Ryukyus, Okinawa, Japan; Laboratory of Parasitology, Department of Disease Control, Graduate School of Veterinary Medicine, Hokkaido University, Sapporo, Japan Abstract. Seventeen Leishmania stocks isolated from cutaneous lesions of Pakistani patients were studied by multi- locus enzyme electrophoresis and by polymerase chain reaction amplification and sequencing of the cytochrome b (Cyt b) gene. Eleven stocks that expressed nine zymodemes were assigned to L. (Leishmania) major. All of them were isolated from patients in the lowlands of Larkana district and Sibi city in Sindh and Balochistan provinces, respectively. The remaining six, distributed in two zymodemes (five and one), isolated from the highland of Quetta city, Balochistan, were identified as L. (L.) tropica. The same result at species level was obtained by the Cyt b sequencing for all the stocks examined. -

International Activity Report 2017
INTERNATIONAL ACTIVITY REPORT 2017 www.msf.org THE MÉDECINS SANS FRONTIÈRES CHARTER Médecins Sans Frontières is a private international association. The association is made up mainly of doctors and health sector workers, and is also open to all other professions which might help in achieving its aims. All of its members agree to honour the following principles: Médecins Sans Frontières provides assistance to populations in distress, to victims of natural or man-made disasters and to victims of armed conflict. They do so irrespective of race, religion, creed or political convictions. Médecins Sans Frontières observes neutrality and impartiality in the name of universal medical ethics and the right to humanitarian assistance, and claims full and unhindered freedom in the exercise of its functions. Members undertake to respect their professional code of ethics and to maintain complete independence from all political, economic or religious powers. As volunteers, members understand the risks and dangers of the missions they carry out and make no claim for themselves or their assigns for any form of compensation other than that which the association might be able to afford them. The country texts in this report provide descriptive overviews of MSF’s operational activities throughout the world between January and December 2017. Staffing figures represent the total full-time equivalent employees per country across the 12 months, for the purposes of comparisons. Country summaries are representational and, owing to space considerations, may not be comprehensive. For more information on our activities in other languages, please visit one of the websites listed on p. 100. The place names and boundaries used in this report do not reflect any position by MSF on their legal status. -

OFFICE of the PRINCIPAL CHANDKA MEDICAL COLLEGE SHAHEED MOHTARMA BENAZIR BHUTO Clo Cl) E
OFFICE OF THE PRINCIPAL CHANDKA MEDICAL COLLEGE SHAHEED MOHTARMA BENAZIR BHUTO MEDICAL UNIVERSITY (SMBBMU) LARKANA, SINDH, PAKISTAN Phont No. (92)4174.9410715 & 9410724 Fax No, (92). 074-9410511 Digit.I're1ilwn. Exhan No.074-9410750 ExI: 311. 312 & 313 . ss-s' & ww'.s '.,t1,mu .du._pk E-ntail inioi iu.edu.14 & j1osmbbrnu.edu.pk - . - .1 . - . - a a - a a - a • - • • NO. CMC/ACCI'S/ c DATED: /2018 To, The Director Information (Advertisement) Public Relation Department. Government of Sindh, Block No.96. Karachi. Subject: NOTICE OF INVITATION TENDER. Five Copies for Invitation of Tender for SMBB Medical University. Larkana, are sent herewith, with the request that the said notice be published in widely circulated daily 'DAWN' Karachi, "JANG" Karachi, "KAWISH" Hyderabad and other newspapers in single insertion. NCIPAL CHANDKA MEDICAL COLLEGE LA RKA NA Copy for Information to: • The Registrar SMBB, Medical University Larkana. • Director Finance, SMBB, Medical University Larkana. • to Vice Chancellor, SMBB Medical University, Larkana. \f Director (A&F) Sindh Public Procurement Regularity Authoi'ity. Government of Sindh Karachi. for SPPRA website. • Incharge website SMBB Medical University, Larkana. • Office record. 6 z E—. cLO Cl) OFFICE OF THE PRINCIPAL CHANDKA MEDICAL COLLEGE SHAHEED MOHTARMA BENAZIR BHUTTO MEDICAL UNiVERSITY (SMBBMU) LARKANA, SINDH, PAKISTAN Phone No. (92).074-9410715 & 9410724 Fax No. (92)- 074-9.110511 Digital Telephone Exchange No.074-9410750 ExI: 311.312 & 313 Website : www.cmc.edu.pk & www.sinithmu.edu.pk E-mail : into nw.edu.pk & [email protected] • a -S • - JS • - NO.CMC/ACCrS/9 ft1 DATED: I'/2O18 NOTICE OF INVITATION TENDER Sealed tenders are invited from the manufactures or repair of Transport only registered with Sales Tax and Income Tax Departments having at least 05 years Experience in related field of Transport and supply of items (repair of Uino Bus) for the Financial Year 2017-20 18 in respect of Chandka Medical College Larkana. -

Issues of Hazara Community and Sectarianism in Quetta (Pakistan)
IMPACT: Journal of Modern Developments in Social Sciences Research (IMPACT: JMDSSR) Vol. 1, Issue 1, Jun 2017, 45-54 © Impact Journals ISSUES OF HAZARA COMMUNITY AND SECTARIANISM IN QUETTA (PAKISTAN) RAB NAWAZ 1 & NAVEED UL HASSAN 2 1Research Scholar, School of Politics and International Relations, Quaid-I-Azam University Islamabad, Pakistan 2Research Scholar, University of Peshawar, Pakistan ABSTRACT Almost 500,000 to 550,000 people of Hazara community of Quetta, Baluchistan are facing sectarianism. Historically the people of Hazara community are peace loving, law abiding and hard working citizens of Pakistan. They are loyal with Pakistan and are serving different departments of Pakistan. The well known banned terrorist organization named Lashkar e Jhangvi claims public responsibility of Hazara Shia killing in Quetta and profess it as a major objective of their organization until annihilation of the Hazara Shia people from Pakistan. Similarly Hazara community facing many other serious problems to protect themselves from terrorism: Inability of Pakistani state to take legal actions against terrorist who does sectarian killings, different government’s tendency to support militant and terrorist organizations and individuals also for so called strategic objectives. Many families migrated not only in other cities of Pakistan like Peshawar and Karachi but also from Pakistan to Australia and other countries. In this situation the Hazara Shia community must do something for self protection and advocacy for their safety with governmental help. In last ten years they had done many efforts through lawful file suits, media awareness for their rights and through other legal tactics. This document focused some incidents of terrorist activities against Hazara Shia community in Quetta. -

A Historical Account of Sectarianism in Pakistan and Persecution of Shia Hazara Community of Quetta in Balochistan
Pakistan Social Sciences Review P-ISSN 2664-0422 March 2021, Vol. 5, No. I [380-392] O-ISSN 2664-0430 RESEARCH PAPER A Historical Account of Sectarianism in Pakistan and Persecution of Shia Hazara Community of Quetta in Balochistan Dr. Gulshan Majeed* Assistant Professor, Department of Political Science , University of the Punjab, Lahore, Punjab, Pakistan PAPER INFO ABSTRACT Received: Pakistan represents a society which is plural in nature with January 23, 2021 different ethnic, religious, cultural and linguistic identities. Accepted: Since its inception Pakistan has been facing a number of March 01, 2021 Online: issues but the issue to manage various religious and ethnic March 15, 2021 minorities emerged as more significant and intricate one. Keywords: After independence ruling authority started to assert Islam Persecution in such a way that religious minorities started to feel Plural, themselves insecure in Pakistan. Shia Hazara community is Religious Minorities, also one among those religious minorities which have been Sectarian Conflicts facing various sectarian conflicting situations. The main *Corresponding objective of this paper is to explain comprehensive historical Author account of sectarianism in Pakistan and reasons of persecution of Shia Hazara community of Quetta. This article is a constructive addition in the existing knowledge related to sectarianism and its impact on society of Pakistan. Researcher has conducted this research by using the gulshan_99@hot historical and descriptive research methods to analyze the mail.com issue and to draw a conclusion. Introduction The emergent orthodoxy with reference to the traditional Islam in Pakistan is closely linked with the rise of militancy, sectarian tendencies and religious othering. -

The Multi-Layered Minority: Exploring the Intersection of Gender, Class and Religious-Ethnic Affiliation in the Marginalisation of Hazara Women in Pakistan
CREID INTERSECTIONS SERIES Religious Inequalities and Gender The Multi-Layered Minority: Exploring the Intersection of Gender, Class and Religious-Ethnic Affiliation in the Marginalisation of Hazara Women in Pakistan Sadiqa Sultan, Maryam Kanwer and Jaffer Abbas Mirza December 2020 Part of the CREID Intersection Series Collection on Violence and Discrimination Against Women of Religious Minority Backgrounds in Pakistan About CREID The Coalition for Religious Equality and Inclusive Development (CREID) provides research evidence and delivers practical programmes which aim to redress poverty, hardship, and exclusion resulting from discrimination on the grounds of religion or belief. CREID is an international consortium led by the Institute of Development Studies (IDS) and funded by UK aid from the UK Government. Key partners include Al-Khoei Foundation, Minority Rights Group (MRG), and Refcemi. Find out more: www.ids.ac.uk/creid. © Institute of Development Studies 2020 Front cover image credit: Surian Soosay CC BY-2.0 ISBN: 978-1-78118-728-9 DOI: 10.19088/CREID.2020.005 This is an Open Access paper distributed under the terms of the Creative Commons Attribution 4.0 International licence (CC BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original authors and source are credited and any modifications or adaptations are indicated. Available from: Coalition for Religious Equality and Inclusive Development (CREID), Institute of Development Studies (IDS), Brighton BN1 9RE, UK Tel: +44(0) 1273 915704 -

College Inspection Report
PAKISTAN MEDICAL COMMISSION LAST RECOGNIZED INSPECTION GRADES OF PRIVATE MEDICAL COLLEGES Sr. Date of Previous Name of Institute City Grade No. Inspection 1. Abbottabad International Medical College Abbottabad. 17-12-2019 A 2. Abwa Medical College Faisalabad 20-11-2018 B 3. Aga Khan University Medical College Karachi. 05-08-2019 A+ 4. Akhtar Saeed Medical & Dental College Lahore. 26-08-2019 A 5. Al Aleem Medical College Lahore 29-08-2019 B 6. Al-Nafees Medical College Islamabad. 30-12-2015 C 7. Al-Tibri Medical College Karachi. 08-11-2013 B 8. Amna Inayat Medical College Lahore. 28-09-2017 C 9. Avicenna Medical College Lahore. 28-08-2019 A 10. Aziz Fatimah Medical & Dental College Faisalabad. 21-03-2018 C 11. Azra Naheed Medical College Lahore. 30-04-2015 B 12. Bahria University Medical College Karachi. 07-08-2019 B 13. Bakhtawar Amin Medical & Dental College Multan 20-02-2016 A 14. Baqai Medical College Karachi. 19-12-2018 F 15. Central Parks Medical College Lahore. 10-02-2015 C 16. CMH Institute of Medical Sciences Bahawalpur Bahawalpur 22-08-2019 C 17. CMH Kharian Medical College Kharian Cantt. 31-07-2019 B 18. CMH Lahore Medical College Lahore Cantt. 30-08-2019 A+ 19. CMH Multan Institute of Medical Sciences CIMS Multan Cantt 20-08-2019 A 20. Continental Medical College Lahore. 16-10-2018 C GRADING CRITERIA: 92.5% or above = A+, 85% or above = A, 77.5% or above = B, 70% or above = C, 69.9% or lower = F Sr. Date of Previous Name of Institute City Grade No. -

Abstract of ICEHSR
7th International Conference of Endorsing Health Science Research – ICEHSR-19 1 7th International Conference of Endorsing Health Science Research – ICEHSR-19 PREFACE International Conference for Endorsing Health Science Research has been a platform since 6 years for researchers from all over the world to exchange their thoughts on research and future developments in health sciences. ICEHSR with its previous impactful scientific history, has now become a brand enhancing the knowledge and information through numerous scientific activities throughout the session including keynote talks, workshops, panel sessions, scientific oral and poster presentations and publications. For the year 2019, team AEIRC is now back with its 7th International Conference for Endorsing Health Science Research (ICEHSR’19) in collaboration with World Health Organization (WHO) with the theme entitled: “Health for All; Everyone Everywhere” at Dow University of Health Sciences, Ojha Campus. AEIRC has been involved in arranging this mega event each year to encourage younger researchers in the field of health sciences by promoting the tradition of research through investigation, questioning and reaching out to appropriate answers. This year ICEHSR has been programmed to bring in the views from different health sectors. The main aim is to indulge the scientists and researchers from biomedical, environmental, psychological, pharmaceutical and all other health domains to identify the social issues and rather than just discussion the focus is break through the barriers and reach out to all possible solutions for it. This abstract book encloses an extracted form of all the sessions/talks delivered by our honorable speakers as well as 161 abstracts those were selected in the conference, presented by one of the author as poster and oral presentations. -

Viral Hepatitis in Pakistan: Past, Present, and Future 1Amna Subhan Butt, 1Fatima Sharif
EJOHG Amna Subhan Butt, Fatima Sharif 10.5005/jp-journals-10018-1172 MINI REVIEW Viral Hepatitis in Pakistan: Past, Present, and Future 1Amna Subhan Butt, 1Fatima Sharif ABSTRACT Viral hepatitis is a major cause of morbidity and mortality worldwide and a rising cause for concern in Asian countries. Weather it is blood borne or water/food borne hepatotropic virus, increasing burden is alarming for Asian countries. In this review we have evaluated the existing data to estimate the burden of viral hepatitis in populations of all age groups nationwide, along with an assessment of the risk factors and preventive and management strategies currently employed in Pakistan. The aim of our work is to consolidate and supplement the present knowledge regarding viral hepatitis in light of past and present trends and to provide future direction to the existing health policies. Keywords: Hepatitis A, Hepatitis B and C, Hepatitis E, Pakistan, Viral hepatitis. How to cite this article: Butt AS, Sharif F. Viral Hepatitis in Pakistan: Past, Present, and Future. Euroasian J Hepato-Gastroenterol 2016;6(1):70-81. Source of support: Nil Conflict of interest: None Copyright and License information: Copyright © 2016; Jaypee Brothers Medical Publishers (P) Ltd. This work is licensed under a Creative Commons Attribution 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by/3.0/ FOOD BORNE PATHOGENS: of acute hepatitis A are much lower than the actual rate HEPATITIS A AND E existing in the population.11 Hepatitis E in Pakistan has been witnessed to occur as Developing countries are at a high risk of infection with outbreaks and sporadic cases in circumstances involving orofecal pathogens. -
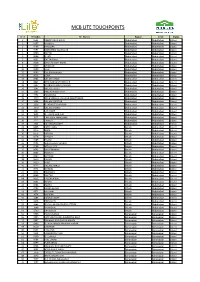
Mcb Lite Touchpoints
MCB LITE TOUCHPOINTS Sr. # Br Codes Br. Names Region Circle Status 1 0585 ABBOTTABAD MAIN Abbottabad Abbottabad Litized 2 0587 HARIPUR Abbottabad Abbottabad Litized 3 0588 HAVELIAN Abbottabad Abbottabad Litized 4 0591 MANSEHRA Star Branch Abbottabad Abbottabad Litized 5 0600 BATTAL Abbottabad Abbottabad Litized 6 0606 GHAZI Abbottabad Abbottabad Litized 7 0622 BATTAGRAM Abbottabad Abbottabad Litized 8 0628 SARAI NIAMAT KHAN Abbottabad Abbottabad Litized 9 0639 SHINKIARI Abbottabad Abbottabad Litized 10 0640 OGHI Abbottabad Abbottabad Litized 11 0643 QALANDARABAD Abbottabad Abbottabad Litized 12 0651 LORA Abbottabad Abbottabad Litized 13 0761 VILLAGE KHAKI Abbottabad Abbottabad Litized 14 0824 NEW DARBAN TOWNSHIP Abbottabad Abbottabad Litized 15 1197 DARBAND ADDA HARIPUR Abbottabad Abbottabad Litized 16 1267 VILLAGE ALLULI Abbottabad Abbottabad Litized 17 1303 PIND KARGOO KHAN Abbottabad Abbottabad Litized 18 1304 KANGRORA Abbottabad Abbottabad Litized 19 1320 AYUB MEDICAL COLLEGE ABBOTTABAD Abbottabad Abbottabad Litized 20 1324 VILLAGE QAZIPUR Abbottabad Abbottabad Litized 21 1358 GHAZIKOT TOWNSHIP Abbottabad Abbottabad Litized 22 1370 VILLAGE JHANGI Abbottabad Abbottabad Litized 23 1387 LAB MORE Abbottabad Abbottabad Litized 24 1655 G.T.ROAD HARIPUR Abbottabad Abbottabad Litized 25 1671 LARI ADDA MANSEHRA Abbottabad Abbottabad Litized 26 1713 THAKOT Abbottabad Abbottabad Litized 27 1724 KALABAGH CANTT Abbottabad Abbottabad Litized 28 0455 FATEH JANG Attock Abbottabad Litized 29 0475 NARA Attock Abbottabad Litized 30 0575 MITHIAL Attock