Textbook Standards
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Socio-Economic & Demographic
SOCIO-ECONOMIC & DEMOGRAPHIC BASELINE STUDY OF PARERAH, DILJABBA AND ARA FOREST COMMUNITIES-CHAKWAL FOREST DIVISION SUSTAINABLE FOREST MANAGEMENT PROJECT Rana Mohammad Farooq Dr. Tahir Mahmood 1 SOCIO-ECONOMIC & DEMOGRAPHIC BASELINE STUDY OF PARERAH, DILJABBA AND ARA FOREST COMMUNITIES-CHAKWAL FOREST DIVISION SUSTAINABLE FOREST MANAGEMENT TO SECURE MULTIPLE BENEFITS IN PAKISTAN’S HIGH CONSERVATION VALUE FORESTS By Rana Mohammad Farooq Dr. Tahir Mahmood 2 Executive Summary The United Nations Development Program (UNDP) and Ministry of Climate Change (MoCC) have launched a project titled “Sustainable forest management to secure multiple benefits in Pakistan’s high conservation value of forests”. The project is aimed at to promote sustainable forest management in Pakistan’s western Himalayan temperate coniferous, subtropical broad leaved evergreen thorn (scrub) and riverine forest for biodiversity conservation, mitigation of climate change and securing of forest ecosystem service. This paper encompasses the study of socio-economic aspects of the salt range scrub forest landscape located in Chakwal and Jhelum Districts of the Punjab province, to develop a framework for sustainable management. The landscape represents an ecosystem that contributes largely to economic and welfare of the communities which depend, directly or indirectly on the exploitation of natural resources of this ecosystem. Hence, there is interrelationship between environment and socio- economic conditions prevailing in the area. They are affected by the natural environment with its various ecosystems which provide continues supply of goods and services. The stability of environment, sustainable forest management and welfare of communities are highly associated and correlated. The total area of landscape is 20,000 ha, out of which 7,859 ha is forested and owned by the state, and 6,672 ha in the outer landscape is private land, including cultivation (1,752 ha) and pastures and settlements, 5469 ha is communal land. -

Project Title to Be Centred
Initial Environmental Examination Conversion of 66kV Danda Shah Bilawal Grid Station to 132kV & New 132kV Double Circuit Feeding Transmission Line February 2017 PAK: MFF – Power Distribution Enhancement Investment Program (Tranche 4) Prepared by Islamabad Electric Supply Company, Federal Capital for the Asian Development Bank. 2 NOTES (i) The fiscal year (FY) of the Government of the Islamic Republic of Pakistan and its agencies ends on 30 June. (ii) In this report “$” refer to US dollars. This Initial Environmental Examination is a document of the Borrower. The views expressed herein do not necessarily represent those of ADB’s Board of Directors, Management, or staff, and may be preliminary in nature. In preparing any country program or strategy, financing any project, or by making any designation of or reference to a particular territory or geographic area in this document, the Asian Development Bank does not intend to make any judgments as to the legal or other status of any territory or area. ISLAMABAD ELECTRIC SUPPLY COMAPNY INITIAL ENVIRONMENTAL EXAMINATION REPORT (IEE) OF Conversion of 66 kV Danda Shah Bilawal Grid Station (to 132 kV) & New 132 KV Double Circuit Feeding Transmission Line POWER DISTRIBUTION ENHANCEMENT PROJECT (PDEIP) LOAN NO.: 3096 – PK TRANCHE - IV SAVINGS SUBPROJECT UNDER ASIAN DEVELOPMENT BANK MULTI TRANCHE FINANCING FACILITY (MFF) February 2017 Prepared & Submitted By ENVIRONMENTAL & SOCIAL SAFEGUARD UNIT OFFICE OF GENERAL MANAGER (DEVELOPMENT) MANAGEMENT UNIT ISLAMABAD ELECTRIC SUPPLY COMPANY (IESCO) ISLAMABAD – PAKISTAN Power Distribution Enhancement Project – Tranche – IV (IESCO) – Saving Subprojects Initial Environmental Examination (IEE) Report TABLE OF CONTENTS 1. INTRODUCTION 1 1.1 Overview. 1 1.2 Need of the Study 2 1.3 Scope of the IEE Study and Personnel. -

Sr. No College Name District Gender Division Contact 1 GOVT
Sr. College Name District Gender Division Contact No 1 GOVT. COLLEGE FOR WOMEN ATTOCK ATTOCK Female RAWALPINDI 572613336 2 GOVT. DEGREE COLLEGE FOR WOMEN FATEH JANG, ATTOCK ATTOCK Female RAWALPINDI 572212505 3 GOVT. COLLEGE FOR WOMEN PINDI GHEB, ATTOCK ATTOCK Female RAWALPINDI 4 GOVT. DEGREE COLLEGE FOR WOMEN, JAND ATTOCK ATTOCK Female RAWALPINDI 572621847 5 GOVT. DEGREE COLLEGE FOR WOMEN HASSAN ABDAL ATTOCK ATTOCK Female RAWALPINDI 6 GOVT. DEGREE COLLEGE FOR WOMEN HAZRO, ATTOCK ATTOCK Female RAWALPINDI 572312884 7 GOVT. POST GRADUATE COLLEGE ATTOCK ATTOCK Male RAWALPINDI 579316163 8 Govt. Commerce College, Attock ATTOCK Male RAWALPINDI 9 GOVT. DEGREE COLLEGE FATEH JANG ATTOCK ATTOCK Male RAWALPINDI 10 GOVT. INTER COLLEGE OF BOYS, BAHTAR, ATTOCK ATTOCK Male RAWALPINDI 11 GOVT. DEGREE COLLEGE (BOYS) PINDI GHEB ATTOCK ATTOCK Male RAWALPINDI 572352909 12 Govt. Institute of Commerce, Pindigheb ATTOCK Male RAWALPINDI 572352470 13 GOVT. DEGREE COLLEGE BOYS, JAND, ATTOCK ATTOCK Male RAWALPINDI 572622310 14 GOVT. INTER COLLEGE NARRAH KANJOOR CHHAB ATTOCK ATTOCK Male RAWALPINDI 572624005 15 GOVT. DEGREE COLLEGE BASAL ATTOCK ATTOCK Male RAWALPINDI 572631414 16 Govt. Institute of Commerce, Jand ATTOCK Male RAWALPINDI 572621186 17 GOVT. DEGREE COLLEGE FOR BOYS HASSAN ABDAL, ATTOCK ATTOCK Male RAWALPINDI 18 GOVT.SHUJA KHANZADA SHAHEED DEGREE COLLEGE, HAZRO, ATTOCK ATTOCK Male RAWALPINDI 572312612 19 GOVT. COLLEGE FOR WOMEN CHAKWAL CHAKWAL Female RAWALPINDI 543550957 20 GOVT. DEGREE COLLEGE FOR WOMEN , DHADIAL , CHAKWAL CHAKWAL Female RAWALPINDI 543590066 21 GOVT. DEGREE COLLEGE FOR WOMEN MULHAL MUGHLAN, CHAKWAL CHAKWAL Female RAWALPINDI 543585081 22 GOVT. DEGREE COLLEGE FOR WOMEN BALKASSAR , CHAKWAL CHAKWAL Female RAWALPINDI 543569888 23 Govt Degree College for women Ara Basharat tehsil choa Saidan Shah chakwal CHAKWAL Female RAWALPINDI 543579210 24 GOVT. -

Final Report
FINAL REPORT CRP 1.1: Dry Land Systems CRP Activity Title: Improving Water and Land Productivity in Rain fed Systems Using Community- based Water and Agronomic Management Approaches. Sub-Activity Title: Capacity building of women of farming community for value-addition to locally-produced fruits and vegetables 1 | P a g e Background Pakistan is an agriculture-based country where majority of the agricultural activities revolve around women, therefore the role of rural women cannot be underestimated or ignored in rural development. The value addition in the form of preservation and processing of agricultural produce has tremendous potential for providing employment and additional income to farm families in rural catchments. Important aspects for promotion of value addition of fruits and vegetables are lower income from sale of fresh commodities in the market, high post-harvest losses due to perishable nature, considerable potential to generate employment, diversify income and improve food security in Pakistan. Increasing production is one approach to improving farm incomes and food availability, but an additional strategy with considerable promise is to add value to agricultural produce. Improved post-harvest handling, processing and marketing achieves value added products and is perhaps the most viable means to reduce poverty and improve rural livelihoods. At initial level, value addition of locally-produced fruits and vegetables for livelihood can develop skills of the rural communities leading to commercialization in the long run as it requires proper certification. Many rural areas that are faced with a declining number of farm jobs consider the food processing sector as a source of potential income and employment growth. -

Population According to Religion, Tables-6, Pakistan
-No. 32A 11 I I ! I , 1 --.. ".._" I l <t I If _:ENSUS OF RAKISTAN, 1951 ( 1 - - I O .PUlA'TION ACC<!>R'DING TO RELIGIO ~ (TA~LE; 6)/ \ 1 \ \ ,I tin N~.2 1 • t ~ ~ I, . : - f I ~ (bFICE OF THE ~ENSU) ' COMMISSIO ~ ER; .1 :VERNMENT OF PAKISTAN, l .. October 1951 - ~........-.~ .1',l 1 RY OF THE INTERIOR, PI'ice Rs. 2 ~f 5. it '7 J . CH I. ~ CE.N TABLE 6.-RELIGION SECTION 6·1.-PAKISTAN Thousand personc:. ,Prorinces and States Total Muslim Caste Sch~duled Christian Others (Note 1) Hindu Caste Hindu ~ --- (l b c d e f g _-'--- --- ---- KISTAN 7,56,36 6,49,59 43,49 54,21 5,41 3,66 ;:histan and States 11,54 11,37 12 ] 4 listricts 6,02 5,94 3 1 4 States 5,52 5,43 9 ,: Bengal 4,19,32 3,22,27 41,87 50,52 1,07 3,59 aeral Capital Area, 11,23 10,78 5 13 21 6 Karachi. ·W. F. P. and Tribal 58,65 58,58 1 2 4 Areas. Districts 32,23 32,17 " 4 Agencies (Tribal Areas) 26,42 26,41 aIIjab and BahawaJpur 2,06,37 2,02,01 3 30 4,03 State. Districts 1,88,15 1,83,93 2 19 4,01 Bahawa1pur State 18,22 18,08 11 2 ';ind and Kbairpur State 49,25 44,58 1,41 3,23 2 1 Districts 46,06 41,49 1,34 3,20 2 Khairpur State 3,19 3,09 7 3 I.-Excluding 207 thousand persons claiming Nationalities other than Pakistani. -

Estimates of Charged Expenditure and Demands for Grants (Development)
GOVERNMENT OF THE PUNJAB ESTIMATES OF CHARGED EXPENDITURE AND DEMANDS FOR GRANTS (DEVELOPMENT) VOL - II (Fund No. PC12037 – PC12043) FOR 2020 - 2021 TABLE OF CONTENTS Demand # Description Pages VOLUME-I PC22036 Development 1 - 968 VOLUME-II PC12037 Irrigation Works 1 - 49 PC12041 Roads and Bridges 51 - 294 PC12042 Government Buildings 295-513 PC12043 Loans to Municipalities / Autonomous Bodies, etc. 515-529 GOVERNMENT OF THE PUNJAB GENERAL ABSTRACT OF DISBURSEMENT (GROSS) (Amount in million) Budget Revised Budget Estimates Estimates Estimates 2019-2020 2019-2020 2020-2021 PC22036 Development 255,308.585 180,380.664 256,801.600 PC12037 Irrigation Works 25,343.061 18,309.413 18,067.690 PC12041 Roads and Bridges 35,000.000 41,510.013 29,820.000 PC12042 Government Buildings 34,348.354 14,827.803 32,310.710 PC12043 Loans to Municipalities/Autonomous Bodies etc. 76,977.253 28,418.359 29,410.759 TOTAL :- 426,977.253 283,446.252 366,410.759 Current / Capital Expenditure detailed below: New Initiatives of SED for imparting Education through (5,000.000) - (4,000.000) Outsourcing of Public Schools (PEIMA) New Initiatives of SED for imparting Education through (19,500.000) - (18,000.000) Private Participation (PEF) Daanish School and Centres of Excellence Authority (1,500.000) - (1,000.000) Punjab Education Endowment Funds (PEEF) (300.000) - (100.000) Punjab Higher Education Commission (PHEC) (100.000) - (50.000) Establishment of General Hospital at Turbat, Baluchistan - - (50.000) Pakistan Kidney & Liver Institute and Research Center (500.000) - -

Estimates of Charged Expenditure and Demands for Grants (Development)
GOVERNMENT OF THE PUNJAB ESTIMATES OF CHARGED EXPENDITURE AND DEMANDS FOR GRANTS (DEVELOPMENT) VOL - I (Fund No. PC22036) FOR 2014 – 2015 TABLE OF CONTENTS Demand # Description Pages VOLUME-I PC22036 Development 1-613 VOLUME-II PC12037 Irrigation Woks 1-51 PC12038 Agricultural Improvement and Research 53-57 PC12040 Town Development 59-63 PC12041 Roads and Bridges 65-203 PC12042 Government Buildings 205-497 PC12043 Loans to Municipalities / Autonomuous Bodies, etc. 499-513 GOVERNMENT OF THE PUNJAB GENERAL ABSTRACT OF DISBURSEMENT (GROSS) (Amount in million) Budget Revised Budget Estimates Estimates Estimates 2013-2014 2013-2014 2014-2015 PC22036 Development 170,705.637 153,460.881 216,595.841 PC12037 Irrigation Works 34,976.500 24,532.612 47,975.188 PC12038 Agricultural Improvement and Research 181.140 171.780 190.551 PC12040 Town Development 500.000 495.858 500.000 PC12041 Roads and Bridges 32,991.000 30,823.709 31,710.000 PC12042 Government Buildings 50,645.723 14,629.754 48,028.420 PC12043 Loans to Municipalities/Autonomous Bodies etc. 10,530.348 14,817.877 13,546.444 TOTAL :- 300,530.348 238,932.471 358,546.444 Current / Capital Expenditure detailed below: TEVTA / TEVTEC (1,500.000) - (2,000.000) Daanish School System (3,000.000) - (2,000.000) PMDGP/PHSRP WB, DFID Sponsored (3,000.000) - (2,000.000) / Vertical Program QA Solar Bahawalpur - - (9,000.000) Punjab Education Endowment Fund (PEEF) (2,000.000) - (2,000.000) Punjab Education Foundation (PEF) (7,500.000) - (7,500.000) Financing of Vertical Program (2,000.000) - - Greend Development -

TMA 14. Chakwal AY 2016-17.Pdf
AUDIT REPORT ON THE ACCOUNTS OF TEHSIL MUNICIPAL ADMINISTRATIONS DISTRICT CHAKWAL AUDIT YEAR 2016-17 AUDITOR GENERAL OF PAKISTAN Table of Contents ABBREVIATIONS AND ACRONYMS .......................................................................... i PREFACE…………. ................................................................................................ii EXECUTIVE SUMMARY ...................................................................................... iii SUMMARY TABLES & CHARTS ............................................................................. vi Table 1: Audit Work Statistics ....................................................................................... vi Table 2: Audit Observations Regarding Financial Management .................................................... vi Table3: Outcome Statistics .......................................................................................... vii Table4: Irregularities Pointed Out ................................................................................... vii Table 5: Cost-Benefit ...............................................................................................viii CHAPTER 1 ......................................................................................................... 1 1.1 TEHSIL MUNICIPAL ADMINISTRATIONS, DISTRICT CHAKWAL .................. 1 1.1.1 Introduction.............................................................................................. 1 1.1.2 Comments on Budget and Accounts (Variance Analysis) ............................................ -

Preparing the Sustainable Livelihood in Barani Areas Project (Punjab), and Hereby Reports This Action to the Board
ASIAN DEVELOPMENT BANK TAR:PAK 34331 TECHNICAL ASSISTANCE (Financed by the Japan Special Fund) TO THE ISLAMIC REPUBLIC OF PAKISTAN FOR PREPARING THE SUSTAINABLE LIVELIHOOD IN BARANI AREAS PROJECT (PUNJAB) September 2003 CURRENCY EQUIVALENTS (as of 31 July 2003) Currency Unit – Pakistan rupee/s (PRe/PRs) PRe1.00 = $0.0174 $1.00 = PRs57.60 ABBREVIATIONS ABAD – Agency for Barani Area Development ADB – Asian Development Bank CBO – community-based organization CCB – citizen community board GIS – geographic information system IEE – initial environmental examination IFAD – International Fund for Agricultural Development IT – information technology NGO – nongovernment organization O&M – operation and maintenance TA – technical assistance NOTES (i) The fiscal year (FY) of the Government of Pakistan ends on 30 June. (ii) In this report, "$" refers to US dollars. This report was prepared by a team consisting of K. Oswald, poverty reduction specialist/team leader; S. Ellison-McGee, and R. Ali. I. INTRODUCTION 1. In 2002, the Government of Pakistan requested project preparatory technical assistance (TA) for a Third Barani Development Project.1 An Asian Development Bank (ADB) Fact-Finding Mission visited Pakistan from 23 April to 3 May 2003 and held meetings with Federal and Punjab provincial government departments and agencies, nongovernment organizations (NGOs), and other aid agencies. Field visits were made to earlier and ongoing barani projects and discussions were also held with district government officials in Chakwal, Gujrat, Jehlum, Narowal, and Sialkot districts. The Mission reached an understanding with the Government regarding the objectives, scope, cost estimates, financing plan, outline terms of reference, and implementation schedule for the TA. II. ISSUES 2. -
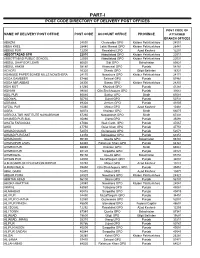
Part-I: Post Code Directory of Delivery Post Offices
PART-I POST CODE DIRECTORY OF DELIVERY POST OFFICES POST CODE OF NAME OF DELIVERY POST OFFICE POST CODE ACCOUNT OFFICE PROVINCE ATTACHED BRANCH OFFICES ABAZAI 24550 Charsadda GPO Khyber Pakhtunkhwa 24551 ABBA KHEL 28440 Lakki Marwat GPO Khyber Pakhtunkhwa 28441 ABBAS PUR 12200 Rawalakot GPO Azad Kashmir 12201 ABBOTTABAD GPO 22010 Abbottabad GPO Khyber Pakhtunkhwa 22011 ABBOTTABAD PUBLIC SCHOOL 22030 Abbottabad GPO Khyber Pakhtunkhwa 22031 ABDUL GHAFOOR LEHRI 80820 Sibi GPO Balochistan 80821 ABDUL HAKIM 58180 Khanewal GPO Punjab 58181 ACHORI 16320 Skardu GPO Gilgit Baltistan 16321 ADAMJEE PAPER BOARD MILLS NOWSHERA 24170 Nowshera GPO Khyber Pakhtunkhwa 24171 ADDA GAMBEER 57460 Sahiwal GPO Punjab 57461 ADDA MIR ABBAS 28300 Bannu GPO Khyber Pakhtunkhwa 28301 ADHI KOT 41260 Khushab GPO Punjab 41261 ADHIAN 39060 Qila Sheikhupura GPO Punjab 39061 ADIL PUR 65080 Sukkur GPO Sindh 65081 ADOWAL 50730 Gujrat GPO Punjab 50731 ADRANA 49304 Jhelum GPO Punjab 49305 AFZAL PUR 10360 Mirpur GPO Azad Kashmir 10361 AGRA 66074 Khairpur GPO Sindh 66075 AGRICULTUR INSTITUTE NAWABSHAH 67230 Nawabshah GPO Sindh 67231 AHAMED PUR SIAL 35090 Jhang GPO Punjab 35091 AHATA FAROOQIA 47066 Wah Cantt. GPO Punjab 47067 AHDI 47750 Gujar Khan GPO Punjab 47751 AHMAD NAGAR 52070 Gujranwala GPO Punjab 52071 AHMAD PUR EAST 63350 Bahawalpur GPO Punjab 63351 AHMADOON 96100 Quetta GPO Balochistan 96101 AHMADPUR LAMA 64380 Rahimyar Khan GPO Punjab 64381 AHMED PUR 66040 Khairpur GPO Sindh 66041 AHMED PUR 40120 Sargodha GPO Punjab 40121 AHMEDWAL 95150 Quetta GPO Balochistan 95151 -

1951-81 Population Administrative . Units
1951- 81 POPULATION OF ADMINISTRATIVE . UNITS (AS ON 4th FEBRUARY. 1986 ) - POPULATION CENSUS ORGANISATION ST ATIS TICS DIVISION GOVERNMENT OF PAKISTAN PREFACE The census data is presented in publica tions of each census according to the boundaries of districts, sub-divisions and tehsils/talukas at the t ime of the respective census. But when the data over a period of time is to be examined and analysed it requires to be adjusted fo r the present boundaries, in case of changes in these. It ha s been observed that over the period of last censuses there have been certain c hanges in the boundaries of so me administrative units. It was, therefore, considered advisable that the ce nsus data may be presented according to the boundary position of these areas of some recent date. The census data of all the four censuses of Pakistan have, therefore, been adjusted according to the administ rative units as on 4th February, 1986. The details of these changes have been given at Annexu re- A. Though it would have been preferable to tabulate the whole census data, i.e., population by age , sex, etc., accordingly, yet in view of the very huge work involved even for the 1981 Census and in the absence of availability of source data from the previous three ce nsuses, only population figures have been adjusted. 2. The population of some of the district s and tehsils could no t be worked out clue to non-availability of comparable data of mauzas/dehs/villages comprising these areas. Consequently, their population has been shown against t he district out of which new districts or rehsils were created. -

Interest Free Loan Programme
INTEREST FREE LOAN PROGRAMME Plan of Cheque Distribution Events of Interest Free Loan (IFL) Programme for September, 2019 Locations of Cheque distribution Contact Details Sr. No. PO Name Province Districts Ceremony (Loan Center with Name of Contact Date of Ceremony Cell Number complete address) Person Akhuwat Islamic 1 Azad Jammu and Kashmir Bagh Bagh (Government College Masjid) Nadeem Ahmed 0314-5273451 26-Sep-19 Microfinance (AIM) Akhuwat Islamic 2 Azad Jammu and Kashmir Bagh Dhir Kot (Jamiya Masjid Dheer kot) Nadeem Ahmed 0314-5273451 24-Sep-19 Microfinance (AIM) Akhuwat Islamic Harighel (Mang bajri arja near 3 Azad Jammu and Kashmir Bagh Nadeem Ahmed 0314-5273451 23-Sep-19 Microfinance (AIM) chambar hotel Harighel) Akhuwat Islamic 4 Azad Jammu and Kashmir Bhimber Bhimber (Jamia Masjid Bimber) Arshad Mehmood 0346-4663605 23-Sep-19 Microfinance (AIM) Akhuwat Islamic 5 Azad Jammu and Kashmir Bhimber Barnala (Jamia Masjid Barnala) Arshad Mehmood 0346-4663605 24-Sep-19 Microfinance (AIM) Akhuwat Islamic Samahni (Main choki Bazar near Sir 6 Azad Jammu and Kashmir Bhimber Arshad Mehmood 0346-4663605 23-Sep-19 Microfinance (AIM) Syed girls College choki Samahni) HHRD District Office Hattian,Near Helping Hand for Relief and Smart Electronics,Choke Bazar, PO, 7 Azad Jammu and Kashmir Hattian Adnan Anwer 0341-9488995 16-Sep-19 Development (HHRD) Tehsil and District Hattianbala. (Hattian) HHRD District Office Langla,Near Helping Hand for Relief and Smart Electronics,Choke Bazar, PO, 8 Azad Jammu and Kashmir Hattian Adnan Anwer 0341-9488995 17-Sep-19 Development (HHRD) Tehsil and District Hattianbala. (Hattian) Helping Hand for Relief and Zahid Hussain HHRD Lamnian office 9 Azad Jammu and Kashmir Hattian Zahid Hussain 0345-9071063 18-Sep-19 Development (HHRD) Main Lamnian Bazar Hattian Bala.