A Menace to Hawaii Nurseries
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sp09-For Web.Pub
Spring 2009 Page 1 Botanic Garden News The Botanic Garden Volume 12, No. 1 of Smith College Spring 2009 Madelaine Zadik “T he tulip is the sexiest, most capricious, the most various, subtle, powerful, and intriguing Room. Many thanks to the Museum of flower on Earth.” These are the words of Anna Art for framing them for us. Pavord, opening speaker for this year’s Spring In our display case are other tulip- Bulb Show. A mainstay of flower shows and related books lent to us by the garden displays, the tulip has come a long way Mortimer Rare Book Room. Flora’s from its humble origins in central Asia to Feast: A Masque of Flowers (1889) is Tulipa ‘Carmen Rio’ becoming a beloved spring icon. Could you opened to the tulip and hyacinth, two of Photograph by Madelaine Zadik imagine spring without tulips? forty full color lithographs in the book Horticulturist and writer extraordinaire Anna Pavord dazzled everyone with her by Walter Crane. Each page presents an talk, The Tulip: The Flower That Made Men Mad. It was more performance than allegory of a popular flower as human, lecture and demonstrated that although tulipmania might have taken over Europe in clad in flowery garments with a short the early seventeenth century, passions today still run quite strong as far as the tulip whimsical verse. Reproductions of is concerned. (For more about Anna Pavord’s visit, see page 7.) During the several of these (see page 6) were tulipmania period in Europe, fortunes rose to soaring heights and then were quickly scattered through the bulb show, to lost, perhaps similar to the Wall Street turbulence we are everyone’s delight. -
Show Activity
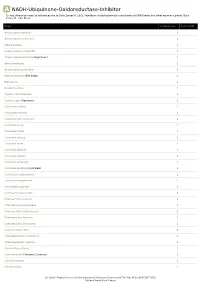
A NADH-Ubiquinone-Oxidoreductase-Inhibitor *Unless otherwise noted all references are to Duke, James A. 1992. Handbook of phytochemical constituents of GRAS herbs and other economic plants. Boca Raton, FL. CRC Press. Plant # Chemicals Total PPM Adenocarpus foliolosus 1 Adenocarpus decorticans 1 Albizia procera 1 Amphicarpaea edgworthii 1 Amphicarpaea bracteata Hog Peanut 1 Apios americana 1 Argyrocytisus battandieri 1 Baptisia tinctoria Wild Indigo 1 Baptisia sp 1 Bowdichia nitida 1 Cajanus scarabaeoides 1 Cajanus cajan Pigeonpea 1 Calicotome villosa 1 Calicotome spinosa 1 Calopogonium caeruleum 1 Camptosema sp 1 Canavalia virosa 1 Canavalia sericea 1 Canavalia rosea 1 Canavalia gladiata 1 Canavalia galeata 1 Canavalia eurycarpa 1 Canavalia ensiformis Jack Bean 1 Centrosema schiedianum 1 Centrosema sagittatum 1 Centrosema plumieri 1 Centrosema pascuorum 1 Chamaecytisus supinus 1 Chamaecytisus smyrnaeus 1 Chamaecytisus ratisbonensis 1 Chamaecytisus hirsutus 1 Chamaecytisus eriocarpus 1 Chamaecytisus albus 1 Chamaespartium tridentatum 1 Chamaespartium sagittale 1 Chronanthus biflorus 1 Cicer arietinum Chickpea; Garbanzo 1 Clitoria ternatea 1 Clitoria falcata 1 Dr. Duke's Phytochemical and Ethnobotanical Databases Downloaded Thu Sep 30 21:42:43 EDT 2021 National Agricultural Library Plant # Chemicals Total PPM Cologana broussonetii 1 Crotalaria juncea Sunhemp 1 Cytisus tribracteolatus 1 Cytisus striatus 1 Cytisus scoparius Scotch Broom 1 Cytisus multiflorus 1 Cytisus ingramii 1 Cytisus ardoini 1 Desmodium gangeticum 1 Dioclea glycinoides -

Island Tropical Foliage, Inc. Larry Barr (305) 245-0010 [email protected] [email protected] Nursery Registration: 47234014
October 22, 2020 CERTIFICATION LIST Nematode Certification Expires: October 23, 2020 TYPE I No. 2447 (Texas and Louisiana) Negative for burrowing and guava root-knot nematodes Island Tropical Foliage, Inc. Larry Barr (305) 245-0010 [email protected] [email protected] Nursery Registration: 47234014 1. Acacia choriophylla – 6”, 10”, 14”, 19” pots; 7, 25, 45, 65, gallon pots 2. Acalypha spp – liners; 1, 2, 3, 7 gallon pots 3. Acoelorrhaphe wrightii - 15, 25 gallon pots 4. Acrostichum danaeifolium – 6”, 10”, 14”, 19” pots 5. Actinidia spp – 6”, 10”, 14” pots; 1, 3 gallon pots; bare roots; un-rooted cuttings 6. Adenium obesum - 8” pots; 1, 2, 3, 7, 15 gallon pots 7. *Adonidia merrillii – 6”, 10”, 14”, 19”, 25” pots; 3, 7, 15, 25, 45 gallon pots 8. Aechmea spp – 1, 3, 7, 15 gallon pots 9. Agapanthus africanus – 1, 3 gallon pots 10. Agave americana – 4”, 6”, 8”, 10", 14" pots; 2, 45 gallon pots 11. Agave angustifolia - 6”, 10”, 14”, 17”,21” pots; 1, 2, 3, 7, 15, 25, 45 gallon pots, 2.5 qt 12. Agave attenuate- 1,2,3,7,15, 25, 45 gallon pots 13. Agave bovicornuta - 6”, 10”, 14”, 17”,21” pots; 1, 2, 3, 7, 15, 25, 45 gallon pots & 2.5 qt 14. Agave celsii - 6”, 10”, 14”, 17”,21” pots; 1, 2, 3, 7, 15, 25, 45 gallon pots & 2.5 qt 15. Agave colorata - 6”, 10”, 14”, 17”,21” pots; 1, 2, 3, 7, 15, 25, 45 gallon pots & 2.5 qt 16. Agave desmettiana – 4”, 6”, 10", 14" pots;1, 7, 15, 45 gallon pots 17. -

Certified Nursery
CERTIFIED NURSERY Hawaiian Tropical Plant Nursery, LLC #BRN: 0444 15-1782 Mikana St., 21st Ave. Keaau, HI 96749 VALID FROM YEAR: 2020 Contact: Steve Starnes PHONE: (808) 966-7466 Date Inspected: 5/20/2020 Island: Hawaii Date Inventory Reviewed: 5/20/2020 Plant Genus Pot Sizes Adenanthera pavonina 4X10 inch pot and 5.5 inch Adenanthera perigrina 4X10 inch & 5.5 inch Adonidia merrillii 5.5 inch square pot Aframomum mildbraedii 5.5 inch square Afzelia quanzensis 4X10 inch pot Aglaia odorata 5.5 inch square pot Aglaia odoratissima 4X10 inch & 5.5 inch Alocasia hybrid 5.5 inch square pot Alocasia longiloba 5.5 inch square pot Aloe microstigma 5.5 inch square pot Alpinia purpurea 5.5 inch square pot Amomum subulatum 5.5 inch square Annona muricata 5.5 inch square pot or 4x4x10 Annona scleroderma 5.5 inch square pot or 4x4x10 Anonna cherimola 5.5 inch square pot or 4x4x10 Anonna montana 5.5 inch square pot or 4x4x10 Anonna reticulata 4X10 inch & 5.5 inch Aphelandra sinclairiana 5.5 inch square Areca catechu 5.5 inch square pot Areca guppyana 5.5 inch square pot Areca macrocalyx 5.5 inch square pot Areca macrocarpa 5.5 inch square pot Areca triandra 5.5 inch square pot Areca vestiaria 5.5 inch square pot Artocarpus altilis (A. camansi) 5.5 inch square Artocarpus heterophyllus 5.5 inch square & 4x4x10 & 2.5x10 Artocarpus integer 5.5 inch square & 4x4x10 & 2.5x10 Artocarpus odoratissimus 5.5 inch square pot or 4x4x10 Artocarpus sericicarpus 5.5 inch square pot Attalea cohune 5.5 inch square pot Azadirachta indica 5.5 inch square pot Baccaurea dulcis 5.5 inch square pot Baccaurea racemosa 5.5 inch square Bactris gasipaes 5.5 inch square pot or 4x4x10 Baikiaea plurijuga 4X10 inch & 5.5 inch Bambusa boniopsis 5.5 inch square pot Bambusa distegia 5.5 inch square pot & 2 gal. -

Rbcl and Legume Phylogeny, with Particular Reference to Phaseoleae, Millettieae, and Allies Tadashi Kajita; Hiroyoshi Ohashi; Yoichi Tateishi; C
rbcL and Legume Phylogeny, with Particular Reference to Phaseoleae, Millettieae, and Allies Tadashi Kajita; Hiroyoshi Ohashi; Yoichi Tateishi; C. Donovan Bailey; Jeff J. Doyle Systematic Botany, Vol. 26, No. 3. (Jul. - Sep., 2001), pp. 515-536. Stable URL: http://links.jstor.org/sici?sici=0363-6445%28200107%2F09%2926%3A3%3C515%3ARALPWP%3E2.0.CO%3B2-C Systematic Botany is currently published by American Society of Plant Taxonomists. Your use of the JSTOR archive indicates your acceptance of JSTOR's Terms and Conditions of Use, available at http://www.jstor.org/about/terms.html. JSTOR's Terms and Conditions of Use provides, in part, that unless you have obtained prior permission, you may not download an entire issue of a journal or multiple copies of articles, and you may use content in the JSTOR archive only for your personal, non-commercial use. Please contact the publisher regarding any further use of this work. Publisher contact information may be obtained at http://www.jstor.org/journals/aspt.html. Each copy of any part of a JSTOR transmission must contain the same copyright notice that appears on the screen or printed page of such transmission. The JSTOR Archive is a trusted digital repository providing for long-term preservation and access to leading academic journals and scholarly literature from around the world. The Archive is supported by libraries, scholarly societies, publishers, and foundations. It is an initiative of JSTOR, a not-for-profit organization with a mission to help the scholarly community take advantage of advances in technology. For more information regarding JSTOR, please contact [email protected]. -

Springtime at Fairchild
spring 2011 Springtime at Fairchild; a treasure trove of Jade published by fairchild tropical botanic garden tropical gourmet foods home décor accessories eco-friendly and fair trade products gardening supplies unique tropical gifts books on tropical gardening, cuisine and more Fern Temple Jar, $240 Photo by Gaby Orihuela/FTBG. Sign up today for The Shop at Fairchild’s frequent shopper program! After you make six merchandise purchases, we’ll add them all up and give you 10% of the total back in rewards dollars to spend in the store. fairchild tropical botanic garden 10901 Old Cutler Road, Coral Gables, FL 33156 • 305.667.1651, ext. 3305 • www.fairchildgarden.org • shop online at www.fairchildonline.com contents CONSERVING EARTH BEHIND THE SCENES FROM SPACE 28 34 Two Men Who Helped the Garden Take Root DEPARTMENTS 5 FROM THE DIRECTOR 8 EVENTS 9 NEWS 11 TROPICAL CUISINE 12 WHAT’S BLOOMING 15 EXPLAINING 19 VIS-A-VIS VOLUNTEERS 27 PLANT SOCIETIES 46 BUG BEAT 49 SOUTH FLORIDA GARDENING SUMMER VEGETABLES 42 51 WISH LIST 52 GIFTS AND DONORS 54 VISTAS 59 WHAT’S IN STORE 60 GARDEN VIEWS 64 FROM THE ARCHIVES 66 CONNECT WITH FAIRCHILD Membership at Fairchild Members of Fairchild Tropical Botanic Garden make a big difference, because they are part of a global community focused on tropical plant conservation and education. Fairchild members support programs in faraway places like Madagascar and Kenya. Fairchild members support conservation and education programs right here in South Florida. In fact, Fairchild’s scientists are leading plant conservation efforts in our local areas and neighborhoods. -

On the Road with Sara Stein ~ Collecting Plants to Save Them
On the Road With Sara Stein ~ Collecting Plants to Save Them A Publication of the American Horticultural Society November/December 1996 $3 .95 L TRULY TWISTED ~ Willows for Small Spaces PORTLAND'S TIMBER PRESS ~ Books for Serious Gardeners MECCA IN OHIO -# Rare Mail-Order Treasures SMART ADDRESSES -# Helpful Sites on the Internet Come Celehrate the American Garaen and the 75th AnniverJary 0/ the American Hortkultural Society in SanFranciJcoApril24-2~ 1997 Our 52nf) Annual Meeting will convene in heautiful San Francuco-the fabled "City by the Bay" wnere the deep voice of foghorns rolling over the seven hills and the clank of (able cars will n~ mind. you that this is a city both new and old, both sopn!istic:aied and fresh, a gateway to fabulous gardens and horticultural surprises. VMit RiilJll with its 16 acres of formal display gardens, and while on the peninsula see several private gardens. We'll cap that day with dinner and a tour of the Thomas Chureh Garden at Sunset magazine headquarters. Next day we'll visit gardens in the hills of the East Bay, including the University of California's botanic garden at Berkeley. We will offer optional visits to the wine country of Napa and Sonoma, as well as a day touring gardens over the Golden Gate Bridge toward the Pacific Coast and then back to the Tiburon area. AJ alwayJ~ our banquet will he a bighligb~ this year featuring a look back at 75 years of the Society's history. COURTESY OF [ !SA GWiTHRIE .. '.., Circle your caletUJar now and make plans to share a fabulous weekend in ~ 1~1; San Francisco with fellow AHS members. -

Strongylodon (Leguminosae-Erythrininae), a Revision Ofth E Genus
WAGENINGEN AGRICULTURAL UNIVERSITY PAPERS 90-8(1991) Strongylodon (Leguminosae-Erythrininae), a revision ofth e genus Shing-FanHuan g Department of Plant Taxonomy WageningenAgricultural University, The Netherlands Dateo fpublicatio n24-4-199 1 Wageningen Wm Agricultural University .TÄI/= fJf dûf BIBLIOTHEEK E4MDBOUWUNIVERSITEI1 87-AGENINGEN Cip-Data Koninklijke Bibliotheek,De n Haag Huang, Shing-Fan Strongylodon (Leguminosae-erythrininae), a revision ofth egenu s/ Shing-Fan Huang.- Wageningen : Agricultural University.- 111.- (Wageningen Agricultu ralUniversit ypapers ,ISS N0169-345 X; 90-8(1991)) Withref. ISBN90-6754-185- 0 NUGI835 Subject heading:Strongylodon . ISBN90-6754-185- 0 NUGI83 5 ISSN016 9 345X © Agricultural UniversityWageningen ,Th eNetherlands ,199 1 No part of this publication, apart from abstract, bibliographic and brief quo tations embodied incritica l reviews,ma yb ereproduced , recorded or published inan yfor m includingprint ,photocopy ,microform , electronico relectromagne ticrecor dwithou twritte n permission from thepublishe r Agricultural Universi ty,P.O .Bo x9101 ,670 0H BWageningen ,th eNetherlands . Printedi nth eNetherland sb yVeenma n Drukkers,Wageninge n Contents Summary VII 1. Introduction 1 2. Materials and methods 2 3. History of the genus Strongylodon 3 4. Characters of the genus Strongylodon 6 4.1 Morphology 6 4.1.1 Branches 6 4.1.2 Stipules 6 4.1.3 Leaves 6 4.1.4 Inflorescence 6 4.1.5 Flower 11 4.1.6 Pod 11 4.1.7 Seed 11 4.1.8 Hilum 14 4.2 Cytology 14 4.3 Phytochemistry 14 4.4 Pollen 14 4.5 Pollination 14 4.6 Dispersal 15 4.7 Habit and habitat 15 5. The relationships of Strongylodon 16 6. -
The Leaflet Vallarta Botanical Gardens AC Curator's Corner
The Leaflet Vallarta Botanical Gardens AC February 2012 Curator’s Corner Volume 2, No. 2 Content: Dear Friends of the Gardens, Have you noticed our new logo? We chose the Tillandsia Curator’s Corner 1 jaliscomonticola to be our ambassador plant for several 6th.Annual Gala reasons. Endemic to just our state of Jalisco, Mexico, the Noche 2 bromeliad is found abundantly in forests all around the de las Luminarias Gardens. (Its name attests to the fact that it is a moun- The Philippine Jade tain lover, and of course much 3 Vine of the Gardens’ appeal is their lovely mountainous set- Volunteer Docent ting.) Specifically adapted and suited to the tropical Training Day Jan. 4 dry forests of our region, this plant thrives during the 2012 summer rains and survives the winter dry season with Tropical Gardening strategies such as the ability to utilize moisture from 5 Q & A mountain dews. Although the small purple humming- bird pollinated flowers are visible only seasonally, the Children of Cruz de Huanacaxtle 6 large red- to yellow-tinted bracts around them remain a showy feature of this distinctive beauty throughout Upcoming Events 7 the year, making it a continual source of pleasure. We thank Claudia Lovera of Mata Ortiz Gallery for her gen- Mission Statement 8 erous donation of the rendering of this logo. For more information on Tillandsias of the area, please see Andy Siekkinen’s article in the December 2011 issue of The Leaflet, which is available on our website. We at the Gardens delight in sharing Mexico´s fascinating botanical treasures with visitors. -

Vertical Gardening Flowering Vines for Florida
Vertical Gardening Flowering Vines for Florida 2008 State Master Gardener Conference Sydney Park Brown Vertical Gardening • Versatile uses • Fast results • Capitalizes on space • “Embraces” garden visitors Sheltering spaces Concept of “Garden Rooms” “Doorways” Transition points between areas of the landscape “Hallway” from one area of the garden to another “Ceilings” for Garden Rooms Transform Fences into Living “Walls” Subtle Screening Not so subtle! Direct traffic - Closed “door” Soften Hard Spaces Useful for Narrow Areas Soften Walls Espalier Containers – Small or Tropical Vines Energy Conservation Food Food and Cover for Wildlife Beneficial Insects on Vines Types of Vines • Climbing • Twining • Clambering/Scrambling Climbing Vines • Have rootlets or adhesive disks • Use on solid upright surfaces (trees, fences, or walls) • Can damage mortar and paint • Difficult to remove Climbing Vines • Climb by means of tendrils • Wind in response to friction • Spread horizontally Twining Vines • Climb by encircling upright support • Most spiral in a counter- clockwise direction • Use on poles & other vertical supports (arbors, pergolas, etc.) http://sciencetrack.blogspot.com/2007/07/twining-motion-of-vines.html Clambering/Sprawling Vines • Sprawl, don’t climb or wind • Must be “helped along” • Ex: Climbing roses, bougainvillea Planting and Maintenance of Vines Plant Two Vines Together Plant Two Together! Examples: aMorning Glory (blooms in AM) with Moon Vine (blooms in PM) aCypress Vine with Gloriosa Lily – Two weak vines that support each other -

A Molecular Phylogeny and New Infrageneric Classification of Mucuna Adans. (Leguminosae-Papilionoideae) Including Insights From
Int. J. Plant Sci. 177(1):76–89. 2016. SYMPOSIUM PAPER q 2015 by The University of Chicago. All rights reserved. 1058-5893/2016/17701-0007$15.00 DOI: 10.1086/684131 A MOLECULAR PHYLOGENY AND NEW INFRAGENERIC CLASSIFICATION OF MUCUNA ADANS. (LEGUMINOSAE-PAPILIONOIDEAE) INCLUDING INSIGHTS FROM MORPHOLOGY AND HYPOTHESES ABOUT BIOGEOGRAPHY Tania M. Moura,1,2,* Mohammad Vatanparast,1,3,† Ana M. G. A. Tozzi,‡ Félix Forest,* C. Melanie Wilmot-Dear,§ Marcelo F. Simon,∥ Vidal F. Mansano,# Tadashi Kajita,† and Gwilym P. Lewis* *Comparative Plant and Fungal Biology Department, Royal Botanic Gardens, Kew, Richmond, Surrey, TW9 3AB, United Kingdom; †Department of Biology, Graduate School of Science, Chiba University, 1-33 Yayoi-cho, Inage-ku, Chiba 263-8-522, Japan; ‡Departamento de Biologia Vegetal, Instituto de Biologia, Universidade Estadual de Campinas, Rua Monteiro Lobato, 255, Cidade Universitária Zeferino Vaz, Barão Geraldo, 13083-862 Campinas, SP, Brazil; §Identification and Naming Department, Royal Botanic Gardens, Kew, Richmond, Surrey, TW9 3AB, United Kingdom; ∥Embrapa Recursos Genéticos e Biotecnologia, Parque Estação Biológica, Avenue W5 Norte, 70770-917 Brasília, DF, Brazil; and #Instituto de Pesquisas Jardim Botânico do Rio de Janeiro, Diretoria de Pesquisas, Rua Pacheco Leão, 915, Jardim Botânico, 22460-030 Rio de Janeiro, RJ, Brazil Editor: Patrick S. Herendeen Premise of research. The genus Mucuna has a pantropical distribution and comprises approximately 105 spe- cies, many of which show great economic value for forage, ornament, and medicine. To date, phylogenetic relationships within Mucuna have not been investigated using molecular data. The aim of this study was to build a phylogenetic framework for Mucuna to address questions about its monophyly, infrageneric relationships, divergence times, and biogeography. -
Show Activity
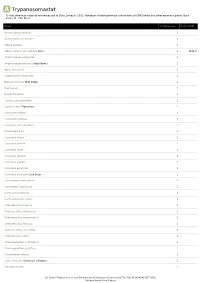
A Trypanosomastat *Unless otherwise noted all references are to Duke, James A. 1992. Handbook of phytochemical constituents of GRAS herbs and other economic plants. Boca Raton, FL. CRC Press. Plant # Chemicals Total PPM Adenocarpus foliolosus 1 Adenocarpus decorticans 1 Albizia procera 1 Allium sativum var. sativum Garlic 1 1931.0 Amphicarpaea edgworthii 1 Amphicarpaea bracteata Hog Peanut 1 Apios americana 1 Argyrocytisus battandieri 1 Baptisia tinctoria Wild Indigo 1 Baptisia sp 1 Bowdichia nitida 1 Cajanus scarabaeoides 1 Cajanus cajan Pigeonpea 1 Calicotome villosa 1 Calicotome spinosa 1 Calopogonium caeruleum 1 Camptosema sp 1 Canavalia virosa 1 Canavalia sericea 1 Canavalia rosea 1 Canavalia gladiata 1 Canavalia galeata 1 Canavalia eurycarpa 1 Canavalia ensiformis Jack Bean 1 Centrosema schiedianum 1 Centrosema sagittatum 1 Centrosema plumieri 1 Centrosema pascuorum 1 Chamaecytisus supinus 1 Chamaecytisus smyrnaeus 1 Chamaecytisus ratisbonensis 1 Chamaecytisus hirsutus 1 Chamaecytisus eriocarpus 1 Chamaecytisus albus 1 Chamaespartium tridentatum 1 Chamaespartium sagittale 1 Chronanthus biflorus 1 Cicer arietinum Garbanzo; Chickpea 1 Clitoria ternatea 1 Dr. Duke's Phytochemical and Ethnobotanical Databases Downloaded Thu Sep 30 16:46:42 EDT 2021 National Agricultural Library Plant # Chemicals Total PPM Clitoria falcata 1 Cologana broussonetii 1 Crotalaria juncea Sunhemp 1 Cytisus tribracteolatus 1 Cytisus striatus 1 Cytisus scoparius Scotch Broom 1 Cytisus multiflorus 1 Cytisus ingramii 1 Cytisus ardoini 1 Desmodium gangeticum 1 Dioclea