Motor and Sensitive Axonal Regrowth After Multiple Intercosto-Lumbar Neurotizations in a Sheep Model
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Effect of the Moufarrege Total Posterior Pedicle Reduction Mammaplasty on the Erogenous Sensation of the Nipple
Surgical Science, 2019, 10, 127-140 http://www.scirp.org/journal/ss ISSN Online: 2157-9415 ISSN Print: 2157-9407 The Effect of the Moufarrege Total Posterior Pedicle Reduction Mammaplasty on the Erogenous Sensation of the Nipple Richard Moufarrege1,2*, Mohammed El Mehdi El Yamani1, Laura Barriault1, Ahmed Amine Alaoui1 1Faculty of Medicine, Université de Montréal, Montreal, Canada 2Department of Plastic Surgery, Université de Montréal, Montreal, Canada How to cite this paper: Moufarrege, R., El Abstract Yamani, M.E.M., Barriault, L. and Alaoui, A.A. (2019) The Effect of the Moufarrege Traditional reduction mammoplasties have the simple concern to guarantee Total Posterior Pedicle Reduction Mam- the survival of the nipple areola complex after surgery. Little has been done to maplasty on the Erogenous Sensation of the take care of essential functions in the nipple, especially the erogenous sensa- Nipple. Surgical Science, 10, 127-140. https://doi.org/10.4236/ss.2019.104016 tion. We have conducted a retrospective study on a cohort of 573 female pa- tients operated using the Total Posterior Pedicle of Moufarrege between 1985 Received: February 25, 2019 and 1995 to evaluate its effect on the erogenous sensation of the nipple. This Accepted: April 23, 2019 study demonstrated the preservation of the erogenous sensation of the nipple Published: April 26, 2019 in a high proportion of these patients. The physiology of this preservation is Copyright © 2019 by author(s) and explained in regard of the technique details in Moufarrege mammoplasty Scientific Research Publishing Inc. compared to other techniques. The Moufarrege Total Posterior Pedicle would This work is licensed under the Creative therefore be a highly reliable reduction technique to ensure the preservation Commons Attribution International License (CC BY 4.0). -

Suggested Osteopathic Treatment.Pdf
Suggested Osteopathic Treatment of Respiratory Diseases Processes Region Biomechanical Model Neurological Model Cardio/Resp Model Metabolic Model Behavioral Model Sample Techniques Head/OA Improve motion CN X - Improve Parasympathetic innervations affect Improve CSF flow (part Reduces anxiety associated with Sub-occipital release; OA decompression; parasympathetic balance heart rate; Improve PRM of PRM) contraction of disease Sinus Drainage (if sings of URI) C-Spine C3-5 Diaphragm C3-5 Diaphragm Assist lymph movement Reduces anxiety associated with Soft Tissue/Myofascial of C-spine, BLT, contraction of disease MET, Counterstrain Thoracic Improve rib cage Stellate Ganglion Lymph drainage (bolster immune Improve oxygenation Normalizes sympathetic drive thus Thoracic Outlet Release, 1st rib release, Outlet motion response) balancing somatopsychological pathways Sternum Improve rib cage Intercostal nerves Improve lymph flow (bolster immune Improve oxygenation Normalizes sympathetic drive thus Sternal/ C-T myofascial release motion response) (reduces work of balancing somatopsychological breathing) pathways Upper Scapula – improve rib Brachial plexus Improve lymph flow Normalizes sympathetic drive thus Scapular balancing, Spencer’s technique, Extremity cage function balancing somatopsychological MET, Counterstrain, Upper Extremity pathways Wobble technique Thoracic Improve rib cage Celiac, Inferior and Improve lymph flow Improve oxygenation Normalizes sympathetic drive thus Soft Tissue/Myofascial of T-spine or Spine motion superior mesenteric -

Intercostal Nerve Conduction Study in Man
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.52.6.763 on 1 June 1989. Downloaded from Journal ofNeurology, Neurosurgery, and Psychiatry 1989;52:763-766 Intercostal nerve conduction study in man SUNIL PRADHAN,* ARUN TALY From the Department ofNeurology, National Institute ofMental Health and Neurosciences, Bangalore, India SUMMARY A new surface technique for the conduction study ofthe lower intercostal nerves has been developed and applied to 30 normal subjects. The problem ofthe short available nerve segment ofthe intercostal nerves and the bizzare compound motor action potential (CMAP) of inconsistent latency while recording over the intercostal muscles, is overcome by applying recording electrodes over the rectus abdominis muscle and stimulating the nerves at two points at a fair distance away. With the use ofmultiple recording sites over the rectus abdominis, the motor points for different intercostal nerves were delineated. CMAP of reproducible latencies and waveforms with sharp take-off points were obtained. Conduction velocity of the intercostal nerves could be determined. There is no standard electrophysiological method of quiet breathing were assured by prior explanation of the studying the nerves of the trunk in man. Even for the procedure. Holding ofbreath was not found necessary for the study. A comma shaped stimulator was placed in the neuropathies which preferentially involve the truncal Protected by copyright. intercostal spaces with the cathode 3 cm anterior to the nerves, for example diabetic thoraco-abdominal anode. It was gently pressed deep and rostral. The intercostal radiculoneuropathy'-3 and segmental zoster nerves were stimulated by a Medelec MS 92 stimulator with a paralysis,45 electrophysiological studies have been supramaximal rectangular pulse of 0 5 ms duration. -

Ultrasound‑Guided Peripheral Nerve Interventions for Common Pain
Published online: 2021-07-26 INTERVENTION RADIOLOGY & VASCULAR Ultrasound‑guided peripheral nerve interventions for common pain disorders Krishna Prasad B P, Binu Joy, Vijayakumar A Raghavendra, Ajith Toms, Danny George, Brijesh Ray1 Department of Radiology, Rajagiri Hospital, Aluva, 1Department of Imaging and Interventional Radiology, Aster Medcity Hospital, Cheranelloor, Ernakulam, Kerala, India Correspondence: Dr. Krishna Prasad B P, Department of Radiology, Rajagiri Hospital, Aluva, Ernakulam - 683 112, Kerala, India. E-mail: [email protected] Abstract There are a number of common pain disorders that can be managed effectively by injections around or ablation of peripheral nerves. Ultrasound is a universally available imaging tool, is safe, cost‑effective, and is excellent in imaging many peripheral nerves and guiding needles to the site of the nerves. This article aims to present an overview of indications and techniques of such procedures that can be effectively performed by a radiologist. Key words: Ganglion block; nerve block; perineural injection Introduction cross‑section, gentle probe tilt ensuring exactly perpendicular orientation of the ultrasound beam will enhance the Peripheral nerve injections have been used for a number of difference in echogenicity between these structures. The common pain causing conditions. Imaging guidance using classic cross‑sectional appearance of the nerves might not be fluoroscopy, computed tomography (CT), or ultrasound apparent when they are very small or deep, in which case, ensures correct site injection; ultrasound among them they are identified by their location and relation to adjacent has a lot of advantages including absence of radiation, more apparent structures. Differentiation of smaller nerves real‑time cross‑sectional visualization of needle placement from blood vessels is made using color Doppler. -

Dorsal Scapular Nerve Neuropathy: a Narrative Review of the Literature Brad Muir, Bsc.(Hons), DC, FRCCSS(C)1
ISSN 0008-3194 (p)/ISSN 1715-6181 (e)/2017/128–144/$2.00/©JCCA 2017 Dorsal scapular nerve neuropathy: a narrative review of the literature Brad Muir, BSc.(Hons), DC, FRCCSS(C)1 Objective: The purpose of this paper is to elucidate Objectif : Ce document a pour objectif d’élucider this little known cause of upper back pain through a cette cause peu connue de douleur dans le haut du narrative review of the literature and to discuss the dos par un examen narratif de la littérature, ainsi que possible role of the dorsal scapular nerve (DSN) in de discuter du rôle possible du nerf scapulaire dorsal the etiopathology of other similar diagnoses in this (NSD) dans l’étiopathologie d’autres diagnostics area including cervicogenic dorsalgia (CD), notalgia semblables dans ce domaine, y compris la dorsalgie paresthetica (NP), SICK scapula and a posterolateral cervicogénique (DC), la notalgie paresthésique (NP), arm pain pattern. l’omoplate SICK et un schéma de douleur postéro- Background: Dorsal scapular nerve (DSN) latérale au bras. neuropathy has been a rarely thought of differential Contexte : La neuropathie du nerf scapulaire dorsal diagnosis for mid scapular, upper to mid back and (NSD) constitue un diagnostic différentiel rare pour la costovertebral pain. These are common conditions douleur mi-scapulaire, costo-vertébrale et au bas/haut presenting to chiropractic, physiotherapy, massage du dos. Il s’agit de troubles communs qui surgissent therapy and medical offices. dans les cabinets de chiropratique, de physiothérapie, de Methods: The methods used to gather articles for this massothérapie et de médecin. paper included: searching electronic databases; and Méthodologie : Les méthodes utilisées pour hand searching relevant references from journal articles rassembler les articles de ce document comprenaient la and textbook chapters. -
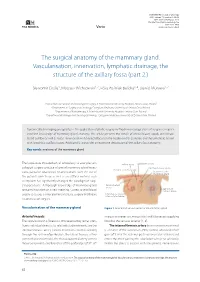
The Surgical Anatomy of the Mammary Gland. Vascularisation, Innervation, Lymphatic Drainage, the Structure of the Axillary Fossa (Part 2.)
NOWOTWORY Journal of Oncology 2021, volume 71, number 1, 62–69 DOI: 10.5603/NJO.2021.0011 © Polskie Towarzystwo Onkologiczne ISSN 0029–540X Varia www.nowotwory.edu.pl The surgical anatomy of the mammary gland. Vascularisation, innervation, lymphatic drainage, the structure of the axillary fossa (part 2.) Sławomir Cieśla1, Mateusz Wichtowski1, 2, Róża Poźniak-Balicka3, 4, Dawid Murawa1, 2 1Department of General and Oncological Surgery, K. Marcinkowski University Hospital, Zielona Gora, Poland 2Department of Surgery and Oncology, Collegium Medicum, University of Zielona Gora, Poland 3Department of Radiotherapy, K. Marcinkowski University Hospital, Zielona Gora, Poland 4Department of Urology and Oncological Urology, Collegium Medicum, University of Zielona Gora, Poland Dynamically developing oncoplasty, i.e. the application of plastic surgery methods in oncological breast surgeries, requires excellent knowledge of mammary gland anatomy. This article presents the details of arterial blood supply and venous blood outflow as well as breast innervation with a special focus on the nipple-areolar complex, and the lymphatic system with lymphatic outflow routes. Additionally, it provides an extensive description of the axillary fossa anatomy. Key words: anatomy of the mammary gland The large-scale introduction of oncoplasty to everyday on- axillary artery subclavian artery cological surgery practice of partial mammary gland resec- internal thoracic artery thoracic-acromial artery tions, partial or total breast reconstructions with the use of branches to the mammary gland the patient’s own tissue as well as an artificial material such as implants has significantly changed the paradigm of surgi- cal procedures. A thorough knowledge of mammary gland lateral thoracic artery superficial anatomy has taken on a new meaning. -

Overview of Spinal Nerves
Spinal Nerves Boundless Overview of Spinal Nerves Spinal nerves, a part of the PNS, generally refers to mixed nerves, with motor, sensory, and autonomic signals between the CNS and the body. 1. fig. 1 shows a spinal nerve Spinal nerves arise from a combination of nerve fibers: the dorsal and ventral roots of the spinal cord. Afferent sensory axons, bringing sensory information from the body to the spinal cord and brain, travel through the dorsal roots of the spinal cord, and efferent motor axons, bringing motor information from the brain to the body, travel through the ventral roots of the spinal cord. All spinal nerves except the first pair emerge from the spinal column through an opening between vertebrae, called an intervertebral foramen. The spinal nerves are typically labeled by their location in the body: thoracic, lumbar, or sacral. Dorsal Root: Also known as the posterior root, the afferent sensory root of a spinal nerve. Autonomic: Acting or occurring involuntarily, without conscious control. Intervertebral Foramen: The foramen allows for the passage of the spinal nerve root, dorsal root ganglion, the spinal artery of the segmental artery, communicating veins between the internal and external plexuses, recurrent meningeal (sinu- vertebral) nerves, and transforaminal ligaments. 2. Source URL: https://www.boundless.com/physiology/peripheral-nervous-system-pns/spinal-nerves/ Saylor URL: http://www.saylor.org/courses/psych402/ Attributed to: [Boundless] www.saylor.org Page 1 of 12 fig. 2 shows intervertebral foramina Intervertebral foramina are indicated by arrows. Spinal Nerves The term spinal nerve generally refers to a mixed spinal nerve, which carries motor, sensory, and autonomic signals between the spinal cord and the body. -

Intercostal, Ilioinguinal, and Iliohypogastric Nerve Transfers for Lower Limb Reinnervation After Spinal Cord Injury: an Anatomical Feasibility and Experimental Study
LABORATORY INVESTIGATION J Neurosurg Spine 30:268–278, 2019 Intercostal, ilioinguinal, and iliohypogastric nerve transfers for lower limb reinnervation after spinal cord injury: an anatomical feasibility and experimental study *Ahmed A. Toreih, MD,1 Asser A. Sallam, MD, PhD,1 Cherif M. Ibrahim, MD,2 Ahmed I. Maaty, MD,3 and Mohsen M. Hassan4 Departments of 1Orthopedic Surgery and Trauma and 3Physical Medicine, Rheumatology, and Rehabilitation, Suez Canal University Hospitals; 2Department of Anatomy, Suez Canal University; and 4Department of Surgery, Anesthesiology, and Radiology, Faculty of Veterinary Medicine, Suez Canal University, Ismailia, Egypt OBJECTIVE Spinal cord injury (SCI) has been investigated in various animal studies. One promising therapeutic ap- proach involves the transfer of peripheral nerves originating above the level of injury into those originating below the level of injury. The purpose of the present study was to evaluate the feasibility of nerve transfers for reinnervation of lower limbs in patients suffering SCI to restore some hip and knee functions, enabling them to independently stand or even step forward with assistive devices and thus improve their quality of life. METHODS The feasibility of transferring intercostal to gluteal nerves and the ilioinguinal and iliohypogastric nerves to femoral nerves was assessed in 5 cadavers. Then, lumbar cord hemitransection was performed below L1 in 20 dogs, followed by transfer of the 10th, 11th, and 12th intercostal and subcostal nerves to gluteal nerves and the ilioinguinal and iliohypogastric nerves to the femoral nerve in only 10 dogs (NT group). At 6 months, clinical and electrophysiological evaluations of the recipient nerves and their motor targets were performed. -

Clinical Policy: Injections and Radiofrequency Neurotomy for Pain Management Reference Number: CP.MP.118 Coding Implications Last Review Date: 04/18 Revision Log
Clinical Policy: Injections and Radiofrequency Neurotomy for Pain Management Reference Number: CP.MP.118 Coding Implications Last Review Date: 04/18 Revision Log See Important Reminder at the end of this policy for important regulatory and legal information. Description Invasive pain management procedures considered in this policy include epidural steroid injections/selective nerve root blocks, facet joint diagnostic and therapeutic blocks and radiofrequency ablation, sacroiliac joint injections and radiofrequency ablation, intradiscal steroid injections, trigger point injections, occipital nerve blocks, peripheral nerve blocks and sympathetic blocks. Policy/Criteria It is the policy of health plans affiliated with Centene Corporation® that invasive pain management procedures performed by a physician are medically necessary when the relevant criteria are met and the patient receives only one procedure per visit, with or without radiographic guidance. I. Caudal or Interlaminar Epidural Steroid Injections .............................................................1 II. Selective Nerve Root Blocks ...............................................................................................2 III. Transforaminal Epidural Steroid Injections .........................................................................3 IV. SNRB/TFESI for Acute Pain Management .........................................................................4 V. Facet Joint Interventions ......................................................................................................4 -

Safe Plastic Surgery of the Breast II: Saving Nipple Sensation
Safe Plastic Surgery of the Breast II: Saving Nipple Sensation Steven Schulz, MD,a,b Matthew R. Zeiderman, MD,c J. Stephen Gunn, MD,a Charles A. Riccio, MD,d Saeed Chowdhry, MD,a,e Ronald Brooks, MD,f Joshua H. Choo, MD,a and Bradon J. Wilhelmi, MDa aThe Ohio State University Department of Plastic Surgery, Columbus, OH; bDivision of Plastic and Reconstructive Surgery, Hiram C. Polk Jr. M.D. Department of Surgery, University of Louisville School of Medicine, Louisville, Ky; cDepartment of Plastic Surgery, University of California Irvine; dDepartment of Plastic Surgery, University of Tennessee Memphis; eChicago Medical School, Rosalind Franklin University of Medicine and Science, Chicago, Ill; and fUniversity of Southern Alabama Plastic and Reconstructive Surgery, Mobile Correspondence: [email protected] Keywords: nipple innervation, reduction mammoplasty, nipple, breast reconstruction, nipple-areola complex Published November 21, 2017 Background: Since its inception, reduction mammoplasty has matured considerably. Primary evolution in clinical research and practice initially focused on developing tech- niques to preserve tissue viability; breast parenchyma, skin, and nipple tissue that has expanded to include sensation and erectile function play a large role in the physical intimacy of women. Studies regarding primary innervation to the nipple are few and often contradictory. Our past anatomical study demonstrated that primary innervation to the nipple to come from the lateral branch of the fourth intercostal nerve. We propose an unsafe zone in which dissection during reduction mammoplasty ought to be avoided to preserve nipple sensation. Objective: To identify the trajectory of innervation to the nipple and translate these findings to the clinical setting so as to preserve nipple sensa- tion. -

Intercostal Nerves Block for Mastectomy in Two Patients with Advanced Breast Malignancy
Intercostal Nerves Block for Mastectomy in Two Patients with Advanced Breast Malignancy Israel K. Kolawole, DA, FWACS; Michael D. Adesina, FWACS; and lyiade 0. Olaoye, FWACS llorin, Nigeria Regional anesthesia is recognized as an altemafive to gener- INTRODUCTION al anesthesia for modern breast cancer sury. Vrious tech- The era of regional anaesthesia dates back to niques of block have ben descrbed. Each has its unique 1884 when Koller discovered the anesthesia proper- problems. Regionl anesthesia was chosen for simple mos- ties of cocaine.' Since then, the scope of regional tectomy in two pafients with'advanced breast malignancy, anesthesia has continued to widen and clinicians due to compromised pulmonary status resulting from wide- have succeeded in gaining access to almost every spread malignant infiltrtfion of both lungs. We used inter- nerve in the body. Consequently, patients who for costalnerves block. The bloc was supplementne with an infr- one reason or another are considered unsuitable for aclicula infiltration to interrupt the branches of the general anesthesia may now have their operations superficiatcervical pexustt provide sensation to the upper done under regional anesthesia. Such was the situa- chest wall and subcutaneous infiltration in the midline to tion with the two patients discussed in this report. block the nerve supply from the contralateral side. Anesthesia Since the breasts are ectodermal organs, which arose was generally effective and the operations were uneveniful. as a modification ofthe sweat glands,2 they -

Intercostal Nerve As a Target for Biopsy Basheer A
Intercostal Nerve As A Target for Biopsy Basheer A. Shakir, MD Khoi Nguyen, MD Haroon F. Choudhri, MD S. Dion Macomson, MD Peripheral Nerve Biopsies . Useful diagnostic tool in peripheral neuropathies, especially when unclear with laboratory, clinical and neurophysiological investigations . Typically sural nerve . Pure sensory nerve – limited usefulness for motor neuropathies or lower motor neuron diseases Peripheral Nerve Biopsy . Techniques for obtaining motor nerve tissue limited in literature and some with risk for motor impairment . Motor branch to gracilis muscle has become a popular target but . unfamiliar anatomy for neurosurgeons . Difficult in obese patient Alternative: Intercostal Nerve . Familiar anatomy . Widely used in neurotization procedures because: . Minimal loss of function from sacrifice . Adequate amount of motor fibers Anatomy of Intercostal Nerves . Mixed peripheral nerve . Easily accessible, familiar anatomy . 1200-1300 fibers, 40 % motor . Somatic nerves arising from anterior divisions of spinal nerves T1-11 . Ventral primary ramus of T12 spinal nerve is subcostal nerve, does not occupy intercostal space Anatomy Intercostal Nerves . Supply thoracic wall, pleura, peritoneum . Intercostal . Typical . Atypical: T1, 2, 7, 8, 9, 10, 11 . Atypical because innervates brachial plexus(1,2), peritoneum (7-11), Anatomy of Intercostal Nerves . Intercostal space 3 layers . External intercostal muscle . Internal intercostal muscle . Innermost intercostal muscle Anatomy of Intercostal Nerve . Upper intercostal nerves (T3, 4, 5, 6) run parallel to their ribs in between internal and innermost muscles . Lower intercostal nerves (T7, 8, 9, 10, 11) lay superficial to transversus thoracic/abdominis muscle Case Series . 4 patients . 2 male, 2 female . Age 26-51 . 3 with preliminary diagnosis of CIDP . One with hereditary polyneuropathy Procedure .