Clinical Policy: Injections and Radiofrequency Neurotomy for Pain Management Reference Number: CP.MP.118 Coding Implications Last Review Date: 04/18 Revision Log
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Simplified Fascial Model of Pelvic Anatomical Surgery: Going Beyond
Anatomical Science International https://doi.org/10.1007/s12565-020-00553-z ORIGINAL ARTICLE A simplifed fascial model of pelvic anatomical surgery: going beyond parametrium‑centered surgical anatomy Stefano Cosma1 · Domenico Ferraioli2 · Marco Mitidieri3 · Marcello Ceccaroni4 · Paolo Zola5 · Leonardo Micheletti1 · Chiara Benedetto1 Received: 13 March 2020 / Accepted: 5 June 2020 © The Author(s) 2020 Abstract The classical surgical anatomy of the female pelvis is limited by its gynecological oncological focus on the parametrium and burdened by its modeling based on personal techniques of diferent surgeons. However, surgical treatment of pelvic diseases, spreading beyond the anatomical area of origin, requires extra-regional procedures and a thorough pelvic anatomical knowl- edge. This study evaluated the feasibility of a comprehensive and simplifed model of pelvic retroperitoneal compartmen- talization, based on anatomical rather than surgical anatomical structures. Such a model aims at providing an easier, holistic approach useful for clinical, surgical and educational purposes. Six fresh-frozen female pelves were macroscopically and systematically dissected. Three superfcial structures, i.e., the obliterated umbilical artery, the ureter and the sacrouterine ligament, were identifed as the landmarks of 3 deeper fascial-ligamentous structures, i.e., the umbilicovesical fascia, the urogenital-hypogastric fascia and the sacropubic ligament. The retroperitoneal areolar tissue was then gently teased away, exposing the compartments delimited by these deep fascial structures. Four compartments were identifed as a result of the intrapelvic development of the umbilicovesical fascia along the obliterated umbilical artery, the urogenital-hypogastric fascia along the mesoureter and the sacropubic ligaments. The retroperitoneal compartments were named: parietal, laterally to the umbilicovesical fascia; vascular, between the two fasciae; neural, medially to the urogenital-hypogastric fascia and visceral between the sacropubic ligaments. -

The Effect of the Moufarrege Total Posterior Pedicle Reduction Mammaplasty on the Erogenous Sensation of the Nipple
Surgical Science, 2019, 10, 127-140 http://www.scirp.org/journal/ss ISSN Online: 2157-9415 ISSN Print: 2157-9407 The Effect of the Moufarrege Total Posterior Pedicle Reduction Mammaplasty on the Erogenous Sensation of the Nipple Richard Moufarrege1,2*, Mohammed El Mehdi El Yamani1, Laura Barriault1, Ahmed Amine Alaoui1 1Faculty of Medicine, Université de Montréal, Montreal, Canada 2Department of Plastic Surgery, Université de Montréal, Montreal, Canada How to cite this paper: Moufarrege, R., El Abstract Yamani, M.E.M., Barriault, L. and Alaoui, A.A. (2019) The Effect of the Moufarrege Traditional reduction mammoplasties have the simple concern to guarantee Total Posterior Pedicle Reduction Mam- the survival of the nipple areola complex after surgery. Little has been done to maplasty on the Erogenous Sensation of the take care of essential functions in the nipple, especially the erogenous sensa- Nipple. Surgical Science, 10, 127-140. https://doi.org/10.4236/ss.2019.104016 tion. We have conducted a retrospective study on a cohort of 573 female pa- tients operated using the Total Posterior Pedicle of Moufarrege between 1985 Received: February 25, 2019 and 1995 to evaluate its effect on the erogenous sensation of the nipple. This Accepted: April 23, 2019 study demonstrated the preservation of the erogenous sensation of the nipple Published: April 26, 2019 in a high proportion of these patients. The physiology of this preservation is Copyright © 2019 by author(s) and explained in regard of the technique details in Moufarrege mammoplasty Scientific Research Publishing Inc. compared to other techniques. The Moufarrege Total Posterior Pedicle would This work is licensed under the Creative therefore be a highly reliable reduction technique to ensure the preservation Commons Attribution International License (CC BY 4.0). -

Suggested Osteopathic Treatment.Pdf
Suggested Osteopathic Treatment of Respiratory Diseases Processes Region Biomechanical Model Neurological Model Cardio/Resp Model Metabolic Model Behavioral Model Sample Techniques Head/OA Improve motion CN X - Improve Parasympathetic innervations affect Improve CSF flow (part Reduces anxiety associated with Sub-occipital release; OA decompression; parasympathetic balance heart rate; Improve PRM of PRM) contraction of disease Sinus Drainage (if sings of URI) C-Spine C3-5 Diaphragm C3-5 Diaphragm Assist lymph movement Reduces anxiety associated with Soft Tissue/Myofascial of C-spine, BLT, contraction of disease MET, Counterstrain Thoracic Improve rib cage Stellate Ganglion Lymph drainage (bolster immune Improve oxygenation Normalizes sympathetic drive thus Thoracic Outlet Release, 1st rib release, Outlet motion response) balancing somatopsychological pathways Sternum Improve rib cage Intercostal nerves Improve lymph flow (bolster immune Improve oxygenation Normalizes sympathetic drive thus Sternal/ C-T myofascial release motion response) (reduces work of balancing somatopsychological breathing) pathways Upper Scapula – improve rib Brachial plexus Improve lymph flow Normalizes sympathetic drive thus Scapular balancing, Spencer’s technique, Extremity cage function balancing somatopsychological MET, Counterstrain, Upper Extremity pathways Wobble technique Thoracic Improve rib cage Celiac, Inferior and Improve lymph flow Improve oxygenation Normalizes sympathetic drive thus Soft Tissue/Myofascial of T-spine or Spine motion superior mesenteric -

Intercostal Nerve Conduction Study in Man
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.52.6.763 on 1 June 1989. Downloaded from Journal ofNeurology, Neurosurgery, and Psychiatry 1989;52:763-766 Intercostal nerve conduction study in man SUNIL PRADHAN,* ARUN TALY From the Department ofNeurology, National Institute ofMental Health and Neurosciences, Bangalore, India SUMMARY A new surface technique for the conduction study ofthe lower intercostal nerves has been developed and applied to 30 normal subjects. The problem ofthe short available nerve segment ofthe intercostal nerves and the bizzare compound motor action potential (CMAP) of inconsistent latency while recording over the intercostal muscles, is overcome by applying recording electrodes over the rectus abdominis muscle and stimulating the nerves at two points at a fair distance away. With the use ofmultiple recording sites over the rectus abdominis, the motor points for different intercostal nerves were delineated. CMAP of reproducible latencies and waveforms with sharp take-off points were obtained. Conduction velocity of the intercostal nerves could be determined. There is no standard electrophysiological method of quiet breathing were assured by prior explanation of the studying the nerves of the trunk in man. Even for the procedure. Holding ofbreath was not found necessary for the study. A comma shaped stimulator was placed in the neuropathies which preferentially involve the truncal Protected by copyright. intercostal spaces with the cathode 3 cm anterior to the nerves, for example diabetic thoraco-abdominal anode. It was gently pressed deep and rostral. The intercostal radiculoneuropathy'-3 and segmental zoster nerves were stimulated by a Medelec MS 92 stimulator with a paralysis,45 electrophysiological studies have been supramaximal rectangular pulse of 0 5 ms duration. -

Anatomical Study of the Superior Cluneal Nerve and Its Estimation of Prevalence As a Cause of Lower Back Pain in a South African Population
Anatomical study of the superior cluneal nerve and its estimation of prevalence as a cause of lower back pain in a South African population by Leigh-Anne Loubser (10150804) Dissertation to be submitted in full fulfilment of the requirements for the degree Master of Science in Anatomy In the Faculty of Health Science University of Pretoria Supervisor: Prof AN Van Schoor1 Co-supervisor: Dr RP Raath2 1 Department of Anatomy, University of Pretoria 2 Netcare Jakaranda Hospital, Pretoria 2017 DECLARATION OF ORIGINALITY UNIVERSITY OF PRETORIA The Department of Anatomy places great emphasis upon integrity and ethical conduct in the preparation of all written work submitted for academic evaluation. While academic staff teach you about referencing techniques and how to avoid plagiarism, you too have a responsibility in this regard. If you are at any stage uncertain as to what is required, you should speak to your lecturer before any written work is submitted. You are guilty of plagiarism if you copy something from another author’s work (e.g. a book, an article, or a website) without acknowledging the source and pass it off as your own. In effect, you are stealing something that belongs to someone else. This is not only the case when you copy work word-for-word (verbatim), but also when you submit someone else’s work in a slightly altered form (paraphrase) or use a line of argument without acknowledging it. You are not allowed to use work previously produced by another student. You are also not allowed to let anybody copy your work with the intention of passing if off as his/her work. -

Ultrasound‑Guided Peripheral Nerve Interventions for Common Pain
Published online: 2021-07-26 INTERVENTION RADIOLOGY & VASCULAR Ultrasound‑guided peripheral nerve interventions for common pain disorders Krishna Prasad B P, Binu Joy, Vijayakumar A Raghavendra, Ajith Toms, Danny George, Brijesh Ray1 Department of Radiology, Rajagiri Hospital, Aluva, 1Department of Imaging and Interventional Radiology, Aster Medcity Hospital, Cheranelloor, Ernakulam, Kerala, India Correspondence: Dr. Krishna Prasad B P, Department of Radiology, Rajagiri Hospital, Aluva, Ernakulam - 683 112, Kerala, India. E-mail: [email protected] Abstract There are a number of common pain disorders that can be managed effectively by injections around or ablation of peripheral nerves. Ultrasound is a universally available imaging tool, is safe, cost‑effective, and is excellent in imaging many peripheral nerves and guiding needles to the site of the nerves. This article aims to present an overview of indications and techniques of such procedures that can be effectively performed by a radiologist. Key words: Ganglion block; nerve block; perineural injection Introduction cross‑section, gentle probe tilt ensuring exactly perpendicular orientation of the ultrasound beam will enhance the Peripheral nerve injections have been used for a number of difference in echogenicity between these structures. The common pain causing conditions. Imaging guidance using classic cross‑sectional appearance of the nerves might not be fluoroscopy, computed tomography (CT), or ultrasound apparent when they are very small or deep, in which case, ensures correct site injection; ultrasound among them they are identified by their location and relation to adjacent has a lot of advantages including absence of radiation, more apparent structures. Differentiation of smaller nerves real‑time cross‑sectional visualization of needle placement from blood vessels is made using color Doppler. -

The Neuroanatomy of Female Pelvic Pain
Chapter 2 The Neuroanatomy of Female Pelvic Pain Frank H. Willard and Mark D. Schuenke Introduction The female pelvis is innervated through primary afferent fi bers that course in nerves related to both the somatic and autonomic nervous systems. The somatic pelvis includes the bony pelvis, its ligaments, and its surrounding skeletal muscle of the urogenital and anal triangles, whereas the visceral pelvis includes the endopelvic fascial lining of the levator ani and the organ systems that it surrounds such as the rectum, reproductive organs, and urinary bladder. Uncovering the origin of pelvic pain patterns created by the convergence of these two separate primary afferent fi ber systems – somatic and visceral – on common neuronal circuitry in the sacral and thoracolumbar spinal cord can be a very dif fi cult process. Diagnosing these blended somatovisceral pelvic pain patterns in the female is further complicated by the strong descending signals from the cerebrum and brainstem to the dorsal horn neurons that can signi fi cantly modulate the perception of pain. These descending systems are themselves signi fi cantly in fl uenced by both the physiological (such as hormonal) and psychological (such as emotional) states of the individual further distorting the intensity, quality, and localization of pain from the pelvis. The interpretation of pelvic pain patterns requires a sound knowledge of the innervation of somatic and visceral pelvic structures coupled with an understand- ing of the interactions occurring in the dorsal horn of the lower spinal cord as well as in the brainstem and forebrain. This review will examine the somatic and vis- ceral innervation of the major structures and organ systems in and around the female pelvis. -

Dorsal Scapular Nerve Neuropathy: a Narrative Review of the Literature Brad Muir, Bsc.(Hons), DC, FRCCSS(C)1
ISSN 0008-3194 (p)/ISSN 1715-6181 (e)/2017/128–144/$2.00/©JCCA 2017 Dorsal scapular nerve neuropathy: a narrative review of the literature Brad Muir, BSc.(Hons), DC, FRCCSS(C)1 Objective: The purpose of this paper is to elucidate Objectif : Ce document a pour objectif d’élucider this little known cause of upper back pain through a cette cause peu connue de douleur dans le haut du narrative review of the literature and to discuss the dos par un examen narratif de la littérature, ainsi que possible role of the dorsal scapular nerve (DSN) in de discuter du rôle possible du nerf scapulaire dorsal the etiopathology of other similar diagnoses in this (NSD) dans l’étiopathologie d’autres diagnostics area including cervicogenic dorsalgia (CD), notalgia semblables dans ce domaine, y compris la dorsalgie paresthetica (NP), SICK scapula and a posterolateral cervicogénique (DC), la notalgie paresthésique (NP), arm pain pattern. l’omoplate SICK et un schéma de douleur postéro- Background: Dorsal scapular nerve (DSN) latérale au bras. neuropathy has been a rarely thought of differential Contexte : La neuropathie du nerf scapulaire dorsal diagnosis for mid scapular, upper to mid back and (NSD) constitue un diagnostic différentiel rare pour la costovertebral pain. These are common conditions douleur mi-scapulaire, costo-vertébrale et au bas/haut presenting to chiropractic, physiotherapy, massage du dos. Il s’agit de troubles communs qui surgissent therapy and medical offices. dans les cabinets de chiropratique, de physiothérapie, de Methods: The methods used to gather articles for this massothérapie et de médecin. paper included: searching electronic databases; and Méthodologie : Les méthodes utilisées pour hand searching relevant references from journal articles rassembler les articles de ce document comprenaient la and textbook chapters. -

Curative Efficacy of Low Frequency Electrical Stimulation in Preventing
Li et al. World Journal of Surgical Oncology (2019) 17:141 https://doi.org/10.1186/s12957-019-1689-2 RESEARCH Open Access Curative efficacy of low frequency electrical stimulation in preventing urinary retention after cervical cancer operation Huan Li1,2†, Can-Kun Zhou4†, Jing Song1,2, Wei-Ying Zhang1,2, Su-Mei Wang1,2, Yi-Ling Gu1,2, Kang Wang1,2, Zhe Ma1,2, Yan Hu1,2, Ai-Min Xiao1,2, Jian-Liu Wang3 and Rui-Fang Wu1,2* Abstract Background: To evaluate the clinical significance of low-frequency electrical stimulation in preventing urinary retention after radical hysterectomy. Methods: A total of 91 women with stage IA2–IB2 cervical cancer, who were treated with radical hysterectomy and lymphadenectomy from January 2009 to December 2012, were enrolled into this study and were randomly divided into two groups: trail group (48 cases) and control group (43 cases). Traditional bladder function training and low- frequency electrical stimulation were conducted in the trail group, while patients in the control group were only treated by traditional bladder training. The general condition, rate of urinary retention, and muscle strength grades of pelvic floor muscle in the perioperative period were compared between these two groups. Results: The incidence of postoperative urinary retention in the electrical stimulation group was 10.41%, significantly lower than that in the control group (44.18%), and the difference was statistically significant (P < 0.01). The duration of postoperative fever and use of antibiotics were almost the same between these two groups. Eleven days after surgery, the difference in grades of the pelvic floor muscle between these two groups was not statistically significant. -
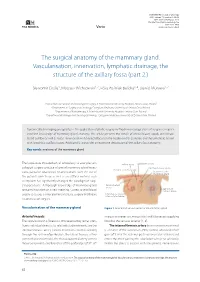
The Surgical Anatomy of the Mammary Gland. Vascularisation, Innervation, Lymphatic Drainage, the Structure of the Axillary Fossa (Part 2.)
NOWOTWORY Journal of Oncology 2021, volume 71, number 1, 62–69 DOI: 10.5603/NJO.2021.0011 © Polskie Towarzystwo Onkologiczne ISSN 0029–540X Varia www.nowotwory.edu.pl The surgical anatomy of the mammary gland. Vascularisation, innervation, lymphatic drainage, the structure of the axillary fossa (part 2.) Sławomir Cieśla1, Mateusz Wichtowski1, 2, Róża Poźniak-Balicka3, 4, Dawid Murawa1, 2 1Department of General and Oncological Surgery, K. Marcinkowski University Hospital, Zielona Gora, Poland 2Department of Surgery and Oncology, Collegium Medicum, University of Zielona Gora, Poland 3Department of Radiotherapy, K. Marcinkowski University Hospital, Zielona Gora, Poland 4Department of Urology and Oncological Urology, Collegium Medicum, University of Zielona Gora, Poland Dynamically developing oncoplasty, i.e. the application of plastic surgery methods in oncological breast surgeries, requires excellent knowledge of mammary gland anatomy. This article presents the details of arterial blood supply and venous blood outflow as well as breast innervation with a special focus on the nipple-areolar complex, and the lymphatic system with lymphatic outflow routes. Additionally, it provides an extensive description of the axillary fossa anatomy. Key words: anatomy of the mammary gland The large-scale introduction of oncoplasty to everyday on- axillary artery subclavian artery cological surgery practice of partial mammary gland resec- internal thoracic artery thoracic-acromial artery tions, partial or total breast reconstructions with the use of branches to the mammary gland the patient’s own tissue as well as an artificial material such as implants has significantly changed the paradigm of surgi- cal procedures. A thorough knowledge of mammary gland lateral thoracic artery superficial anatomy has taken on a new meaning. -

Overview of Spinal Nerves
Spinal Nerves Boundless Overview of Spinal Nerves Spinal nerves, a part of the PNS, generally refers to mixed nerves, with motor, sensory, and autonomic signals between the CNS and the body. 1. fig. 1 shows a spinal nerve Spinal nerves arise from a combination of nerve fibers: the dorsal and ventral roots of the spinal cord. Afferent sensory axons, bringing sensory information from the body to the spinal cord and brain, travel through the dorsal roots of the spinal cord, and efferent motor axons, bringing motor information from the brain to the body, travel through the ventral roots of the spinal cord. All spinal nerves except the first pair emerge from the spinal column through an opening between vertebrae, called an intervertebral foramen. The spinal nerves are typically labeled by their location in the body: thoracic, lumbar, or sacral. Dorsal Root: Also known as the posterior root, the afferent sensory root of a spinal nerve. Autonomic: Acting or occurring involuntarily, without conscious control. Intervertebral Foramen: The foramen allows for the passage of the spinal nerve root, dorsal root ganglion, the spinal artery of the segmental artery, communicating veins between the internal and external plexuses, recurrent meningeal (sinu- vertebral) nerves, and transforaminal ligaments. 2. Source URL: https://www.boundless.com/physiology/peripheral-nervous-system-pns/spinal-nerves/ Saylor URL: http://www.saylor.org/courses/psych402/ Attributed to: [Boundless] www.saylor.org Page 1 of 12 fig. 2 shows intervertebral foramina Intervertebral foramina are indicated by arrows. Spinal Nerves The term spinal nerve generally refers to a mixed spinal nerve, which carries motor, sensory, and autonomic signals between the spinal cord and the body. -

The Spinal Cord and Spinal Nerves
14 The Nervous System: The Spinal Cord and Spinal Nerves PowerPoint® Lecture Presentations prepared by Steven Bassett Southeast Community College Lincoln, Nebraska © 2012 Pearson Education, Inc. Introduction • The Central Nervous System (CNS) consists of: • The spinal cord • Integrates and processes information • Can function with the brain • Can function independently of the brain • The brain • Integrates and processes information • Can function with the spinal cord • Can function independently of the spinal cord © 2012 Pearson Education, Inc. Gross Anatomy of the Spinal Cord • Features of the Spinal Cord • 45 cm in length • Passes through the foramen magnum • Extends from the brain to L1 • Consists of: • Cervical region • Thoracic region • Lumbar region • Sacral region • Coccygeal region © 2012 Pearson Education, Inc. Gross Anatomy of the Spinal Cord • Features of the Spinal Cord • Consists of (continued): • Cervical enlargement • Lumbosacral enlargement • Conus medullaris • Cauda equina • Filum terminale: becomes a component of the coccygeal ligament • Posterior and anterior median sulci © 2012 Pearson Education, Inc. Figure 14.1a Gross Anatomy of the Spinal Cord C1 C2 Cervical spinal C3 nerves C4 C5 C 6 Cervical C 7 enlargement C8 T1 T2 T3 T4 T5 T6 T7 Thoracic T8 spinal Posterior nerves T9 median sulcus T10 Lumbosacral T11 enlargement T12 L Conus 1 medullaris L2 Lumbar L3 Inferior spinal tip of nerves spinal cord L4 Cauda equina L5 S1 Sacral spinal S nerves 2 S3 S4 S5 Coccygeal Filum terminale nerve (Co1) (in coccygeal ligament) Superficial anatomy and orientation of the adult spinal cord. The numbers to the left identify the spinal nerves and indicate where the nerve roots leave the vertebral canal.