Chapter 4 Biological Environment Chapter 5 Human Environment Appendices
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ocean Shore Management Plan
Ocean Shore Management Plan Oregon Parks and Recreation Department January 2005 Ocean Shore Management Plan Oregon Parks and Recreation Department January 2005 Oregon Parks and Recreation Department Planning Section 725 Summer Street NE Suite C Salem Oregon 97301 Kathy Schutt: Project Manager Contributions by OPRD staff: Michelle Michaud Terry Bergerson Nancy Niedernhofer Jean Thompson Robert Smith Steve Williams Tammy Baumann Coastal Area and Park Managers Table of Contents Planning for Oregon’s Ocean Shore: Executive Summary .......................................................................... 1 Chapter One Introduction.................................................................................................................. 9 Chapter Two Ocean Shore Management Goals.............................................................................19 Chapter Three Balancing the Demands: Natural Resource Management .......................................23 Chapter Four Balancing the Demands: Cultural/Historic Resource Management .........................29 Chapter Five Balancing the Demands: Scenic Resource Management.........................................33 Chapter Six Balancing the Demands: Recreational Use and Management .................................39 Chapter Seven Beach Access............................................................................................................57 Chapter Eight Beach Safety .............................................................................................................71 -

Oregon Coast Trail
OREGON COAST TRAIL Finalized Design Submittal Boardman Trail Project Logo - Oregon Coast Trail Submitted by Denise Dahn, Dahn Design 2/1/04 Actual size variable OREGON COAST TRAIL OREGON COAST TRAIL OREGON COAST TRAIL OREGON COAST TRAIL TRAIL TRAIL COAST COAST COAST OREGON COAST TRAIL OREGON OREGON TRAIL OREGON OREGON COAST TRAIL OREGON COAST TRAIL The Oregon Coast Trail begins its 382-mile route at the 1 Columbia River south jetty. The trailhead is 4 miles north of 1. Columbia River to Fort Stevens State Park campground. The first 16 miles is on the beach between the south jetty and Gearhart. Finalized Design Submittal Boardman Trail Project Logo - Oregon Coast Trail Submitted by Denise Dahn, Dahn Design 2/1/04 OREGON Actual size variable Oswald West State Park COAST OREGON COAST TRAIL OREGON COAST TRAIL OREGON COAST TRAIL TRAIL OREGON COAST TRAIL L T AIL RAI AS COLUMBIA RIVER T TR T S L E G E N D CO OA C COAST Fort Stevens EGON OREGON COAST TRAIL OR OREGON TRAIL OREGON OREGON State Park COAST TRAIL OREGON COAST Oregon Coast Trail TRAIL OREGON Beach Trail COAST TRAIL ASTORIA OREGON COAST 30 Trail on Road/Hard Surface TRAIL Alternate Route 101 OREGON COAST TRAIL Roads Finalized Design Submittal Boardman Trail Project Logo - Oregon Coast Trail 104 Submitted by Denise Dahn, Dahn Design 2/1/04 Actual size variable WARRENTON 101B 1 Trail Direction Information OREGON COAST TRAIL OREGON COAST TRAIL OREGON COAST TRAIL OREGON State Park Boundary COAST TRAIL TRAIL TRAIL AST COAST COAST CO OREGON COAST TRAIL OREGON OREGON TRAIL OREGON 104 S OREGON COAST TRAIL Interpretive Exhibit Information OREGON COAST TRAIL 101B Camping AIL 0 1.25 2.5 N A TR miles miles -TO-SE RT FO OREGON COAST TRAIL PLEASE NOTE: The trail route may change due to Sunset Beach safety issues, road closures or State Recreation Site OREGON COAST TRAIL detours. -

Timing of In-Water Work to Protect Fish and Wildlife Resources
OREGON GUIDELINES FOR TIMING OF IN-WATER WORK TO PROTECT FISH AND WILDLIFE RESOURCES June, 2008 Purpose of Guidelines - The Oregon Department of Fish and Wildlife, (ODFW), “The guidelines are to assist under its authority to manage Oregon’s fish and wildlife resources has updated the following guidelines for timing of in-water work. The guidelines are to assist the the public in minimizing public in minimizing potential impacts to important fish, wildlife and habitat potential impacts...”. resources. Developing the Guidelines - The guidelines are based on ODFW district fish “The guidelines are based biologists’ recommendations. Primary considerations were given to important fish species including anadromous and other game fish and threatened, endangered, or on ODFW district fish sensitive species (coded list of species included in the guidelines). Time periods were biologists’ established to avoid the vulnerable life stages of these fish including migration, recommendations”. spawning and rearing. The preferred work period applies to the listed streams, unlisted upstream tributaries, and associated reservoirs and lakes. Using the Guidelines - These guidelines provide the public a way of planning in-water “These guidelines provide work during periods of time that would have the least impact on important fish, wildlife, and habitat resources. ODFW will use the guidelines as a basis for the public a way of planning commenting on planning and regulatory processes. There are some circumstances where in-water work during it may be appropriate to perform in-water work outside of the preferred work period periods of time that would indicated in the guidelines. ODFW, on a project by project basis, may consider variations in climate, location, and category of work that would allow more specific have the least impact on in-water work timing recommendations. -

Paleoethnobotany of Kilgii Gwaay: a 10,700 Year Old Ancestral Haida Archaeological Wet Site
Paleoethnobotany of Kilgii Gwaay: a 10,700 year old Ancestral Haida Archaeological Wet Site by Jenny Micheal Cohen B.A., University of Victoria, 2010 A Thesis Submitted in Partial Fulfillment of the Requirements for the Degree of MASTER OF ARTS in the Department of Anthropology Jenny Micheal Cohen, 2014 University of Victoria All rights reserved. This thesis may not be reproduced in whole or in part, by photocopy or other means, without the permission of the author. Supervisory Committee Paleoethnobotany of Kilgii Gwaay: A 10,700 year old Ancestral Haida Archaeological Wet Site by Jenny Micheal Cohen B.A., University of Victoria, 2010 Supervisory Committee Dr. Quentin Mackie, Supervisor (Department of Anthropology) Dr. Brian David Thom, Departmental Member (Department of Anthropology) Dr. Nancy Jean Turner, Outside Member (School of Environmental Studies) ii Abstract Supervisory Committee Dr. Quentin Mackie, Supervisor (Department of Anthropology) Dr. Brian David Thom, Departmental Member (Department of Anthropology) Dr. Nancy Jean Turner, Outside Member (School of Environmental Studies) This thesis is a case study using paleoethnobotanical analysis of Kilgii Gwaay, a 10,700- year-old wet site in southern Haida Gwaii to explore the use of plants by ancestral Haida. The research investigated questions of early Holocene wood artifact technologies and other plant use before the large-scale arrival of western redcedar (Thuja plicata), a cultural keystone species for Haida in more recent times. The project relied on small- scale excavations and sampling from two main areas of the site: a hearth complex and an activity area at the edge of a paleopond. The archaeobotanical assemblage from these two areas yielded 23 plant taxa representing 14 families in the form of wood, charcoal, seeds, and additional plant macrofossils. -

Humboldt Bay Fishes
Humboldt Bay Fishes ><((((º>`·._ .·´¯`·. _ .·´¯`·. ><((((º> ·´¯`·._.·´¯`·.. ><((((º>`·._ .·´¯`·. _ .·´¯`·. ><((((º> Acknowledgements The Humboldt Bay Harbor District would like to offer our sincere thanks and appreciation to the authors and photographers who have allowed us to use their work in this report. Photography and Illustrations We would like to thank the photographers and illustrators who have so graciously donated the use of their images for this publication. Andrey Dolgor Dan Gotshall Polar Research Institute of Marine Sea Challengers, Inc. Fisheries And Oceanography [email protected] [email protected] Michael Lanboeuf Milton Love [email protected] Marine Science Institute [email protected] Stephen Metherell Jacques Moreau [email protected] [email protected] Bernd Ueberschaer Clinton Bauder [email protected] [email protected] Fish descriptions contained in this report are from: Froese, R. and Pauly, D. Editors. 2003 FishBase. Worldwide Web electronic publication. http://www.fishbase.org/ 13 August 2003 Photographer Fish Photographer Bauder, Clinton wolf-eel Gotshall, Daniel W scalyhead sculpin Bauder, Clinton blackeye goby Gotshall, Daniel W speckled sanddab Bauder, Clinton spotted cusk-eel Gotshall, Daniel W. bocaccio Bauder, Clinton tube-snout Gotshall, Daniel W. brown rockfish Gotshall, Daniel W. yellowtail rockfish Flescher, Don american shad Gotshall, Daniel W. dover sole Flescher, Don stripped bass Gotshall, Daniel W. pacific sanddab Gotshall, Daniel W. kelp greenling Garcia-Franco, Mauricio louvar -

2017-2018 Fishing in Washington Sport Fishing Rules Pamphlet
Sport Fishing Rules Pamphlet Corrections and Updates July 1, 2017 through June 30, 2018 Last updated June 28, 2017. Marine Area Rules Page 98, LANDING A FISH - A club or dipnet (landing net) may be used to assist landing a legal fish taken by legal gear. A gaff may only be used to land a legally hooked LINGCOD (in Marine Areas 1-3 and 4 West of Bonilla-Tatoosh line), HALIBUT, TUNA, or DOGFISH SHARK that will be retained. HALIBUT may be shot or harpooned while landing. Photo By Scott Mayfield General Information Washington Department of Fish & Wildlife (WDFW) Dr. Jim Unsworth, Director Ron Warren, Assistant Director, Fish Program Contents General Information General Washington Fish & Wildlife Commission GENERAL RULES & INFORMATION Dr. Bradley Smith, Chair, Bellingham Jay Kehne, Omak Contact Information ..................................2 Larry Carpenter, Vice Chair, Mount Vernon Miranda Wecker, Naselle Update From WDFW ................................3 Barbara Baker, Olympia Kim Thorburn, Spokane Statewide General Rules .........................4 Jay Holzmiller, Anatone David Graybill, Leavenworth Salmon and Trout Handling Rules ............5 Rules Robert “Bob” Kehoe, Seattle License Information ...............................6-7 Catch Record Cards .................................8 Freshwater Catch Record Card Codes .......................9 How to Use This Pamphlet Definitions ..........................................10-11 FRESHWATER GENERAL RULES This pamphlet is effective July 1, 2017 through June 30, 2018 Statewide Freshwater Rules..............13-15 and contains information you need to legally fish throughout RIVERS .............................................17-73 Washington State (see WAC summary information below). Special Rules Introduction ..................17 Puget Sound Puget Puget Sound and Coast Rivers - Rivers & Coast 1 Read the General Information Pages. Special Rules ...................................18-46 Read the Licensing and Catch Record Card information. -

Busting Broom with Help from Ewe
Celebrating 30 Years NORTH COAST LAND CONSERVANCY SPRING 2016 BUSTING BROOM WITH HELP FROM EWE PacificLight Images If North Coast Land Conservancy’s goal is to “WE’RE PUTTING attempt broom removal using a “forestry conserve habitat for wildlife on the Oregon mulcher”—a large tractor that employs Coast, why are sheep grazing on NCLC’s OUR ALL INTO THIS a rotary drum with steel chipper teeth biggest preserve in the Clatsop Plains? AND HOPING FOR designed to shred vegetation. The forestry Better you should ask, what happened to all THE BEST.” mulcher succeeded in eradicating the last the Scotch broom? mature Scotch broom and the blackberry the dunes. Over several years we managed thickets while leaving native plants such as Sometimes it takes unexpected partners— to eradicate mature broom from most of Nootka rose and willow intact. in this case, a fourth-generation Clatsop the property. However the two easternmost Plains rancher—and tools ranging from dune ridges were so steep and the infestation Another challenge remained: how to keep heavy equipment to nibbling sheep for us to was so large that the usual methods of the broom from returning. Scotch broom achieve our habitat restoration goals. broom removal, including hand cutting, seeds can remain viable in the soil for up weed whacking, and mowing, had proved to 30 years. Annual mowing, which keeps When we purchased 117-acre Reed Ranch— ineffective or impossible. broom in check elsewhere on Reed Ranch, west of US Highway 101 near Cullaby isn’t possible on these steep slopes. So Lake—in 2008, invasive Scotch broom Then in November, we used habitat we arranged with our neighbor, Bill Reed, covered the property, crowding out native restoration funds from the federal Natural to graze sheep on the property. -

Eulachon – a Fish Unique to Our West Coast
Eulachon – A Fish Unique to Our West Coast Eulachon (Thaleichthys pacificus) – also known as candlefish, oolichan, hooligan, salvation fish, or Columbia River smelt - are only found in marine waters, coastal rivers, and estuaries from northern California to Alaska. Because of their high oil content and nutritional value, Eulachon provide important food for many fishes, birds, and marine mammals. They are historically, economically, and culturally significant as a resource for indigenous peoples of the Pacific Northwest, and also support limited and strictly regulated non- tribal fisheries. Eulachon populations in California, Washington, and Oregon are listed as threatened under the Endangered Species Act. Eulachon Distribution Note the adipose fin – all true smelt have one Photo: Jen Zamon, NOAA Life Cycle Eulachon are anadromous - they hatch in Fraser River freshwater rivers, migrate to live in the ocean for several Adult Eulachon, 195 mm. Ridges (striations) on the gill cover years, return to freshwater to spawn, and die after (operculum) are an identifying mark spawning. Adults are broadcast spawners; they spread eggs and milt over fine sands but do not construct nests (redds). One female produces an average of 32,000 tiny, Photo: Jen Zamon, NOAA 1 mm (<1/10th of an inch) eggs. Eulachon eggs stick to Elwha River sand grains, and depending on river temperatures, hatch Threats to Eulachon Survival in 20-40 days. The 4-7mm (~ ¼ inch) hatchlings (larvae) Quileute River are flushed downstream by river flow. Fish live on their The most serious threats to Eulachon survival include yolk sac reserves for about 5 days. With luck, after 5 days • rapid climate change in ocean and freshwater habitats, they reach the estuary and can begin feeding on small • damming and water diversion, and Quinault River diatoms (microscopic plants) before they enter the ocean. -

A Preliminary Status Review of Eulachon and Pacific Lamprey in the Klamath River Basin
A preliminary status review of eulachon and Pacific lamprey in the Klamath River Basin Yurok Tribal Fisheries Program 159OO Hwy 101 N Klamath CA 95548 Zachary S. Larson Michael R. Belchik April I998 INTRODUCTION Eulachon (Thaleicthys pacificus) and Pacific lamprey (Lampetra tridentata) are two species of anadromous fish found throughout the northwestern United States and western Canada that have received little attention relative to salmonid species. Consequently, with few exceptions, very little is known of their status or population trends. Eulachon are one of several species of smelts (Osmeridae) that occur off the coast of California. Their spawning migration takes them further into freshwater inlets than any other smelt within their range. They are prized by many tribes of the Pacific Northwest for their taste, and have been tied to tribal culture for centuries. Pacific lamprey are also harvested and considered a delicacy by tribes of the Pacific Northwest; however, lamprey migrate further up rivers and tributaries to spawn than do eulachon, often utilizing habitat hundreds of miles inland from the ocean (Scott and Crossman 1973). On the Klamath River of northwest California (Fig. l), eulachon and Pacific lamprey are of great importance to the Yurok Tribe but runs have diminished in the past few decades and no efforts have been made to determine factors contributing to apparent declines. Eulachon have apparently disappeared in the Klamath River and other nearby coastal drainages -- only a handful of fish have been witnessed since 1988 (CDFG unpublished data 1988-89, YTFP 1998). Pacific lamprey have exhibited a more gradual decline but little quantitative evidence is available. -
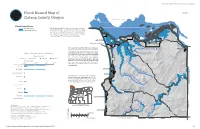
Plate 5. Flood Hazard Map of Clatsop County, Oregon, Appendix E Map
Natural Hazard Risk Report for Clatsop County, Oregon G E O L O G Y F A N O D T N M I E N M E T R R A A L PLATE 5 P I E N Flood Hazard Map of D D U N S O T G R E I R E S O Clatsop County, Oregon WASHINGTON 1937 Flood Hazard Zone 100-Year Flood (1% annual chance) Columbia River sourcesThe �lood include hazard riverine. data show Areas areas are consistentexpected to with be inundated during a 100-year �lood event. Flooding Counties Digital Flood Insurance Rate Maps. the regulatory �lood zones depicted in Clatsop Astoria ¤£101 30 Warrenton «¬104 ¤£ Skipanon River Svensen-Knappa Disclaimer: This product is for informational purposes and may not have been prepared for or be suitable for John Day River Westport legal, engineering, or surveying purposes. Users of this information should review or consult the primary Wallooskee River Ra�o of Es�mated Loss to Flooding data and information sources to ascertain the usability Flood Scenarios of the information. This publication cannot substitute 10-Year 50-Year 100-Year 500-Year ¤£101 for site-speci�ic investigations by quali�ied Exposure Ratio differ from the results shown in the publication. See thepractitioners. accompanying Site-speci�ic text report data for may more give details results on that the ~ ~ «¬202 0% 0.5% 1% 4.5% limitations of the methods and data used to prepare Clatsop County this publication. (rural)* Y o Arch Cape* Gearhart u ng s Ri Svensen-Knappa* ver Seaside Lewis a This map is an overview map and not intended to nd Westport* C er provide details at the community scale. -
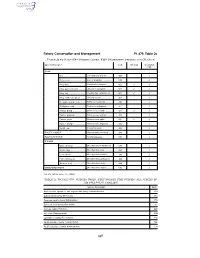
Species Codes: Fmp Prohibited Species and Cr Crab
Fishery Conservation and Management Pt. 679, Table 2c TABLE 2b TO PART 679—SPECIES CODES: FMP PROHIBITED SPECIES AND CR CRAB Species Description Code CR Crab Groundfish PSC CRAB Box ....................................... Lopholithodes mandtii .......... 900 ✓ Dungeness ........................... Cancer magister .................. 910 ✓ King, blue ............................. Paralithodes platypus .......... 922 ✓ ✓ King, golden (brown) ........... Lithodes aequispinus ........... 923 ✓ ✓ King, red .............................. Paralithodes camtshaticus ... 921 ✓ ✓ King, scarlet (deepsea) ....... Lithodes couesi .................... 924 ✓ Korean horsehair crab ......... Erimacrus isenbeckii ............ 940 ✓ Multispinus crab ................... Paralomis multispinus .......... 951 ✓ Tanner, Bairdi ...................... Chionoecetes bairdi ............. 931 ✓ ✓ Tanner, grooved .................. Chionoecetes tanneri ........... 933 ✓ Tanner, snow ....................... Chionoecetes opilio ............. 932 ✓ ✓ Tanner, triangle ................... Chionoecetes angulatus ...... 934 ✓ Verrilli crab ........................... Paralomis verrilli .................. 953 ✓ PACIFIC HALIBUT Hippoglossus stenolepis ...... 200 ✓ PACIFIC HERRING Family Clupeidae ................. 235 ✓ SALMON Chinook (king) ..................... Oncorhynchus tshawytscha 410 ✓ Chum (dog) .......................... Oncorhynchus keta .............. 450 ✓ Coho (silver) ........................ Oncorhynchus kisutch ......... 430 ✓ Pink (humpback) ................. -

Fishery Conservation and Management Pt. 679, Table 2D
Fishery Conservation and Management Pt. 679, Table 2d Chum (dog) .......................... Oncorhynchus keta .............. 450 ✓ Coho (silver) ........................ Oncorhynchus kisutch ......... 430 ✓ Pink (humpback) .................. Oncorhynchus gorbuscha .... 440 ✓ Sockeye (red) ...................... Oncorhynchus nerka ........... 420 ✓ STEELHEAD TROUT Oncorhynchus mykiss ......... 540 ✓ [73 FR 76172, Dec. 15, 2008] TABLE 2c TO PART 679—SPECIES CODES: FMP FORAGE FISH SPECIES (ALL SPECIES OF THE FOLLOWING FAMILIES) Species Description Code Bristlemouths, lightfishes, and anglemouths (family Gonostomatidae) 209 Capelin smelt (family Osmeridae) 516 Deep-sea smelts (family Bathylagidae) 773 Eulachon smelt (family Osmeridae) 511 Gunnels (family Pholidae) 207 Krill (order Euphausiacea) 800 Laternfishes (family Myctophidae) 772 Pacific sandfish (family Trichodontidae) 206 Pacific sand lance (family Ammodytidae) 774 Pricklebacks, war-bonnets, eelblennys, cockscombs and shannys (family Stichaeidae) 208 Surf smelt (family Osmeridae) 515 [70 FR 75083, Dec. 19, 2005] TABLE 2d TO PART 679—SPECIES CODES—NON-FMP SPECIES General use Species description Code Arctic char, anadromous ............................................................................................................................................... 521 Dolly varden, anadromous ............................................................................................................................................ 531 Eels or eel-like fish .......................................................................................................................................................