Laboratory Procedure Manual
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

WELLNESS SCREENING TEST EXPLANATION SHEET We Encourage You to Share All Lab Results with Your Primary Care Provider
WELLNESS SCREENING TEST EXPLANATION SHEET We encourage you to share all lab results with your primary care provider. EXPLANATION OF BLOOD TESTS HEMOGLOBIN A1C: A Hemoglobin A1c level is HEMATOLOGY WELLNESS: expressed as a percent and is used to evaluate the Blood counts are often used as a broad screening test average amount of glucose in the blood over the last to determine an individual’s general health status. 2 to 3 months. This test may be used to screen for and White blood cells are the cells that are part of the diagnose diabetes or risk of developing diabetes. body’s defense system against infections and cancer and also play a role in allergies and inflammation. ALT: This test is used to determine if a patient has Red blood cells are the cells that transport oxygen liver damage. In healthy individuals, ALT levels in the throughout the body. Hemoglobin measures the blood are low and they rise in various disease states. total amount of the oxygen-carrying protein in the blood, which generally reflects the number of red CALCIUM: A calcium test is ordered to screen for, blood cells in the blood. Hematocrit measures the diagnose and monitor a range of conditions percentage of a person’s total blood volume that relating to the bones, heart, nerves, kidneys and consists of red blood cells. Platelets are cell fragments teeth. The test may also be ordered if a person has that are vital for normal blood clotting. symptoms of parathyroid disorder, malabsorption or an overactive thyroid. FREE T4: Free T4 is used to help evaluate thyroid function, generally after determining the TSH is CREATININE: The creatinine blood test is used to abnormal. -

Evaluation of Abnormal Liver Chemistries
ACG Clinical Guideline: Evaluation of Abnormal Liver Chemistries Paul Y. Kwo, MD, FACG, FAASLD1, Stanley M. Cohen, MD, FACG, FAASLD2, and Joseph K. Lim, MD, FACG, FAASLD3 1Division of Gastroenterology/Hepatology, Department of Medicine, Stanford University School of Medicine, Palo Alto, California, USA; 2Digestive Health Institute, University Hospitals Cleveland Medical Center and Division of Gastroenterology and Liver Disease, Department of Medicine, Case Western Reserve University School of Medicine, Cleveland, Ohio, USA; 3Yale Viral Hepatitis Program, Yale University School of Medicine, New Haven, Connecticut, USA. Am J Gastroenterol 2017; 112:18–35; doi:10.1038/ajg.2016.517; published online 20 December 2016 Abstract Clinicians are required to assess abnormal liver chemistries on a daily basis. The most common liver chemistries ordered are serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase and bilirubin. These tests should be termed liver chemistries or liver tests. Hepatocellular injury is defined as disproportionate elevation of AST and ALT levels compared with alkaline phosphatase levels. Cholestatic injury is defined as disproportionate elevation of alkaline phosphatase level as compared with AST and ALT levels. The majority of bilirubin circulates as unconjugated bilirubin and an elevated conjugated bilirubin implies hepatocellular disease or cholestasis. Multiple studies have demonstrated that the presence of an elevated ALT has been associated with increased liver-related mortality. A true healthy normal ALT level ranges from 29 to 33 IU/l for males, 19 to 25 IU/l for females and levels above this should be assessed. The degree of elevation of ALT and or AST in the clinical setting helps guide the evaluation. -

Total Bilirubin in Athletes, Determination of Reference Range
OriginalTotal bilirubin Paper in athletes DOI: 10.5114/biolsport.2017.63732 Biol. Sport 2017;34:45-48 Total bilirubin in athletes, determination of reference range AUTHORS: Witek K1, Ścisłowska J2, Turowski D1, Lerczak K1, Lewandowska-Pachecka S2, Corresponding author: Pokrywka A3 Konrad Witek Department of Biochemistry, Institute of Sport - National 1 Department of Biochemistry, Institute of Sport - National Research Institute, Warsaw, Poland Research Institute 2 01-982 Warsaw, Poland Faculty of Pharmacy with the Laboratory Medicine Division, Medical University of Warsaw, Poland 3 E-mail: Department of Biochemistry, 2nd Faculty of Medicine, Medical University of Warsaw, Poland [email protected] ABSTRACT: The purpose of this study was to determine a typical reference range for the population of athletes. Results of blood tests of 339 athletes (82 women and 257 men, aged 18-37 years) were retrospectively analysed. The subjects were representatives of different sports disciplines. The measurements of total bilirubin (BIT), iron (Fe), alkaline phosphatase (ALP), alanine aminotransferase (ALT) and gamma-glutamyltransferase (GGT) were made using a Pentra 400 biochemical analyser (Horiba, France). Red blood cell count (RBC), reticulocyte count and haemoglobin concentration measurements were made using an Advia 120 haematology analyser (Siemens, Germany). In groups of women and men the percentage of elevated results were similar at 18%. Most results of total bilirubin in both sexes were in the range 7-14 μmol ∙ L-1 (49% of women and 42% of men). The highest results of elevated levels of BIT were in the range 21-28 μmol ∙ L-1 (12% of women and 11% of men). There was a significant correlation between serum iron and BIT concentration in female and male athletes whose serum total bilirubin concentration does not exceed the upper limit of the reference range. -

Normal Rangesа–А
Effective August 2007 5361 NW 33 rd Avenue Fort Lauderdale, FL 33309 954-777-0018 NORMAL RANGES – fax: 954-777-0211 ADULT MALE FEMALE CBC WBC 3.6 – 11.0 K/UL 3.6 – 11.0 RBC 4.5 – 5.9 M/UL 4.5 – 5.9 HEMOGLOBIN 13.0 – 18.0 G/DL 12.0 – 15.6 HEMATOCRIT 40 – 52 % 36.0 – 46.0 MCV 81 – 97 FL 81 93 MCH 26 – 34 PG 26 34 MCHC 31 – 37 gm/dl 31 37 RDW 11.5 – 15 % 11.5 – 15.0 PLATELET CT 150 – 400 K/UL 140 400 PLT VOL 7.4 – 10.4 FL 7.4 – 10.4 NEUTROPHIL % 36 – 66 36 75 LYMPHOCYTE % 23 – 43 27 47 MONOCYTE % 0.0 – 10 0.0 10 EOSINOPHIL % 0 – 5.0 0.0 – 5.0 BASOPHIL % 0 – 1.0 0.0 – 1.0 RETIC 0.4 – 1.9 0.4 – 1.9 COMPREHENSIVE METABOLIC PROFILE /LIVER PROFILE SODIUM 135 – 145 MMOL/L 135 145 POTASSIUM 3.5 – 5.5 MMOL/L 3.5 – 5.5 (OUTREACH) CHLORIDE 95 – 110 MMOL/L 95 110 CO2 19 – 34.MMOL/L 19 34 GLUCOSE 70 – 110 MG/DL 70 110 BUN 6 – 22 MG/DL 6 22 CREATININE 0.6 – 1.3 MG/DL 0.6 – 1.3 MG/DL CALCIUM 8.4 – 10.2 MG/DL 8.4 – 10.2 TOTAL PROTEIN 5.5 – 8.7 G/DL 5.5 – 8.7 ALBUMIN 3.2 – 5.0 g/dl 3.2 – 5.0 BILI TOTAL 0.1 – 1.2 MG/DL 0.1 – 1.2 BILI DIRECT 0.0 – 0.3 MG/DL 0.0 – 0.3 BILI INDIRECT 0.2 – 1.0 MG/DL 0.2 – 1.0 ALKALINE PHOS 20 – 130 U/L 20 130 AST/SGOT 10 – 40 U/L 10 40 ALT/SGPT 7 55 U/L 7 55 AST/ALT RATIO 0.5 – 0.9 RATIO 0.5 – 0.9 AMYLASE 25 – 115 U/L 25 – 115 U/L LIPASE 114 – 286 U/L 114 – 286 U/L CRP 0 – 0.9 MG/DL 0 – 0.9 MG/DL PREALBUMIN 18.0 – 35.7 mg/dL 18.0 – 35.7 mg/dL Revised 807 MAGNESIUM 1.8 – 2.4 mg/dL 1.8 – 2.4 mg/Dl LDH 100 – 200 Units /L 100 – 200 Units/L PHOSPHOROUS 2.3 – 5.0 mg/dL 2.3 – 5.0 mg/dL LACTIC ACID 0.4 -
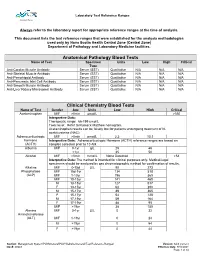
Laboratory Test Reference Ranges
Laboratory Test Reference Ranges Central Zone Always refer to the laboratory report for appropriate reference ranges at the time of analysis. This document lists the test reference ranges that were established for the analysis methodologies used only by Nova Scotia Health Central Zone (Central Zone) Department of Pathology and Laboratory Medicine facilities. Anatomical Pathology Blood Tests Name of Test Specimen Units Low High Critical Type Anti-Cardiac Muscle Antibody Serum (SST) Qualitative N/A N/A N/A Anti-Skeletal Muscle Antibody Serum (SST) Qualitative N/A N/A N/A Anti-Pemphigoid Antibody Serum (SST) Qualitative N/A N/A N/A Anti-Pancreatic Islet Cell Antibody Serum (SST) Qualitative N/A N/A N/A Anti-Smooth Muscle Antibody Serum (SST) Qualitative N/A N/A N/A Anti-Liver Kidney Microsomal Antibody Serum (SST) Qualitative N/A N/A N/A Clinical Chemistry Blood Tests Name of Test Gender Age Units Low High Critical Acetaminophen M/F >0min µmol/L >350 Interpretive Data: Therapeutic range: 66-199 umol/L Toxic level: Refer to Rumack Matthew nomogram. Acetaminophen results can be falsely low for patients undergoing treatment of N- acetylcysteine (NAC). Adrenocorticotropic M/F >0min pmol/L 2.3 10.1 hormone Interpretive Data: Adrenocorticotropic Hormone (ACTH) reference ranges are based on (ACTH) samples collected prior to 10 AM. Albumin M/F 0-1yr g/L 25 46 >1yr 35 50 Alcohol M/F >0min mmol/L None Detected >54 Interpretive Data: The method is intended for clinical purposes only. Medical-legal specimens should be analyzed by gas chromatographic method for confirmation of results. -

Diagnosis of Insulinoma in a Maine Coon
JOR0010.1177/2055116919894782Journal of Feline Medicine and Surgery Open ReportsGifford et al 894782research-article2020 Case Report Journal of Feline Medicine and Surgery Open Reports Diagnosis of insulinoma in 1 –10 © The Author(s) 2020 Article reuse guidelines: a Maine Coon cat sagepub.com/journals-permissions DOI:https://doi.org/10.1177/2055116919894782 10.1177/2055116919894782 journals.sagepub.com/home/jfmsopenreports This paper was handled and processed by the American Editorial Office (AAFP) 1 2 3 Carol H Gifford , Anita P Morris , Kurt J Kenney and for publication in JFMS Open Reports J Scot Estep4 Abstract Case summary A 9-year-old male neutered Maine Coon cat presented with a 6-month history of polyphagia and one recent episode of tremors and weakness. Blood work revealed profound hypoglycemia and results of a paired insulin glucose test were consistent with an insulinoma. Abdominal ultrasound revealed a solitary pancreatic mass, and results of a fine-needle aspirate (FNA) gave further support for the location of the neuroendocrine tumor. After unsuccessful medical management of the hypoglycemia, the mass was surgically removed. Immunohistochemistry confirmed that it was an insulinoma. At the time of writing, the patient had been in clinical remission for 9 months. Relevance and novel information Feline insulinomas are rare and there is very little information on their behavior, clinical course and histologic characteristics. This is the first reported case of an insulinoma in a Maine Coon cat and the first to describe results of an ultrasound-guided FNA of the mass. In addition, the progression of disease, histopathology and immunohistochemistry results add to the currently minimal database for feline insulinomas. -

Levels of Serum Uric Acid in Patients with Impaired Glucose Tolerance
JOURNAL OF CRITICAL REVIEWS ISSN- 2394-5125 VOL 08, ISSUE 02, 2021 LEVELS OF SERUM URIC ACID IN PATIENTS WITH IMPAIRED GLUCOSE TOLERANCE 1,2Shahzad Ali Jiskani*, 1Lubna Naz, 3Ali Akbar Pirzado, 4Dolat Singh, 5Asadullah Hanif, 6Razia Bano 1Department of Pathology, Chandka Medical College, SMBBMU, Larkano 2Department of Pathology, Indus Medical College Hospital, Tando Muhammad Khan 3Department of Community Medicine and Public Health, Chadka Medical College, SMBBMU, Larkana 4Department of Medicine, Indus Medical College Hospital, Tando Muhammad Khan 5Health Department, Govt. of Sindh 6Department of Medicine, Liaquat University of Medical & Health Sciences Jamshoro *Corresponding Author: Shahzad Ali Jiskani Department of Pathology, Chandka Medical College, SMBBMU, Larkano ABSTRACT Objective: The main objective of this study is to see levels of serum uric acid in patients having impaired glucose tolerance as compared to normal glucose tolerant individuals. Patients and Methods: This was prospective study, conducted at Department of Medicine and Department of Pathology, Indus Medical College Hospital, Tando Muhammad Khan for the period of 8 months (March 2019 to October 2019). A total of 140 patients were included in this study. An oral glucose test was performed with load of 75g glucose in patients having impaired glucose in fasting. Both normal glucose tolerance and impaired glucose tolerance individuals were evaluated for their serum uric acid level. Serum glucose levels and serum uric acid levels were performed on COBAS C-111 Chemistry Analyzer ensuring procedures of quality control. Data was entered and analyzed using SPSS 21.0. Results: In patients having impaired glucose tolerance, the frequency of hyperuricemia was 62.35%. Analysis of both groups (patients with normal glucose tolerance and glucose intolerance), association was strong with p-value of <0.001. -

Determination of Reference Ranges for Selected Clinical Laboratory Tests for a Medical Laboratory in Namibia Using Pre-Tested Data
DETERMINATION OF REFERENCE RANGES FOR SELECTED CLINICAL LABORATORY TESTS FOR A MEDICAL LABORATORY IN NAMIBIA USING PRE-TESTED DATA by CORNELIA DE WAAL-MILLER Student no: 197084516 Thesis submitted in fulfillment of the requirements of the degree Master of Technology: Biomedical Technology in the Faculty of Health and Wellness Sciences at the Cape Peninsula University of Technology Supervisor: Professor A.J. Esterhuyse Co-Supervisor: Professor B. Noden Bellville March 2015 CPUT copyright information The thesis may not be published either in part (in scholarly, scientific or technical journals), or as a whole (as a monograph), unless permission has been obtained from the University. (i) Declaration I, Cornelia de Waal-Miller, declare that the content of this thesis represents my own unaided work, and that this thesis has not previously been submitted for academic examination towards any qualification. Furthermore, this thesis represents my own opinions and not necessarily those of the Cape Peninsula University of Technology. 12th March 2015 Signed Date 2 | of 84 Pages (ii) Abstract Aim: The aim of the study was to compile pre-tested laboratory results stored in the laboratory database of the Namibia Institute of Pathology (NIP). The study also aimed to assess the usefulness and validity of using retrospective laboratory results of different patients in varying degrees of health and which were produced using various methods in different laboratories in Namibia. Methods: 254,271 test results (female: 134,261, male = 117,091, unknown gender= 2,919) consisting of Haemoglobin, serum Urea, serum Creatinine, plasma Glucose (fasting and random), serum Cholesterol, serum Triglycerides and serum Uric Acid was extracted from NIP Laboratory Information System over a period of four years and of the 13 different regions of Namibia were analyzed. -

C-Reactive Protein and Gamma-Glutamyltransferase
Hindawi Publishing Corporation Experimental Diabetes Research Volume 2010, Article ID 761715, 8 pages doi:10.1155/2010/761715 Clinical Study C-Reactive Protein and Gamma-Glutamyltransferase Concentrations in Relation to the Prevalence of Type 2 Diabetes Diagnosed by Glucose or HbA1c Criteria in Chinese Adults in Qingdao, China J. Ren,1 Z. C. Pang,2 W. G. Gao,3, 4, 5 H. R. Nan,6 S. J. Wang,2 L. Zhang,3, 4, 5 and Q. Qiao3, 4 1 Department of Epidemiology and Health Statistics, Shandong University, Jinan 250012, China 2 Department of Non-Communicable Disease Prevention, Qingdao Municipal Centre for Disease Control and Prevention, no. 175, Shandong Road, Qingdao 266071, China 3 Department of Public Health, University of Helsinki, 00014 Helsinki, Finland 4 Diabetes Unit, Department of Chronic Disease Prevention, National Institute for Health and Welfare, 00014 Helsinki, Finland 5 Qingdao Endocrinology and Diabetes Hospital, 266071 Qingdao, China 6 Hong Kong Institute of Diabetes and Obesity, Hong Kong, China CorrespondenceshouldbeaddressedtoZ.C.Pang,[email protected] Received 13 August 2010; Revised 12 October 2010; Accepted 12 October 2010 Academic Editor: Giuseppe Paolisso Copyright © 2010 J. Ren et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Aims. To investigate the association of C-reactive protein (CRP) and gamma glutamyltransferase (GGT) concentrations with newly diagnosed diabetes defined by either glucose or HbA1c criteria in Chinese adults. Methods. A population-based cross-sectional study was conducted in 2006. Data from 1167 men and 1607 women aged 35–74 years were analyzed. -

Understanding Your Blood Test Lab Results
Understanding Your Blood Test Lab Results A comprehensive "Health Panel" has been designed specifically to screen for general abnormalities in the blood. This panel includes: General Chemistry Screen or (SMAC), Complete Blood Count or (CBC), and Lipid examination. A 12 hour fast from all food and drink (water is allowed) is required to facilitate accurate results for some of the tests in this panel. Below, is a breakdown of all the components and a brief explanation of each test. Abnormal results do not necessarily indicate the presence of disease. However, it is very important that these results are interpreted by your doctor so that he/she can accurately interpret the findings in conjunction with your medical history and order any follow-up testing if needed. The Bernards Township Health Department and the testing laboratory cannot interpret these results for you. You must speak to your doctor! 262 South Finley Avenue Basking Ridge, NJ 07920 www.bernardshealth.org Phone: 908-204-2520 Fax: 908-204-3075 1 Chemistry Screen Components Albumin: A major protein of the blood, albumin plays an important role in maintaining the osmotic pressure spleen or water in the blood vessels. It is made in the liver and is an indicator of liver disease and nutritional status. A/G Ratio: A calculated ratio of the levels of Albumin and Globulin, 2 serum proteins. Low A/G ratios can be associated with certain liver diseases, kidney disease, myeloma and other disorders. ALT: Also know as SGPT, ALT is an enzyme produced by the liver and is useful in detecting liver disorders. -
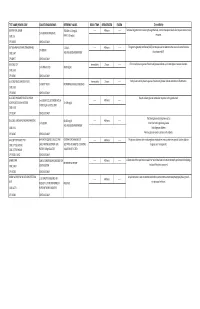
G's- Lab Test Menu
TEST NAME/ PSYCHE CODE COLLECTION GUIDELINES REFERENCE VALUES ROOM TEMP. REFRIGERATED FROZEN Clinical Utility GENTAMICIN, SERUM TROUGH: 0-2.0 mg/dL ------ 48 hours ------ Tests level of gentamicin circulating through the body. Certain therapeutic levels elicit require anti-microbial 2 ml SERUM. REFRIGERATE. CODE: GE PEAK: 5-10 mg/dL response. CPT: 81070 SCHEDULE: DAILY GGT (GAMMA GLUTAMYL TRANSFERASE) 5-31 IU/L ------ 48 hours ------ The gamma-glutamyl transferase (GGT) test may be used to determine the cause of elevated alkaline 1ml SERUM CODE: GGT AGE AND GENDER DEPENDENT phosphatase (ALP). CPT:82977 SCHEDULE: DAILY GLUCOSE, CSF Immediately 2 hours ------ CFS normally has no glucose. Presence of glucose indicates spinal meningitis or seizure disorders. 1 ml SPINAL FLUID 40-80 mg/mL CODE: GLFL CPT: 82947 SCHEDULE: DAILY GLUCOSE, MISCELLANEOUS FLUID Immediately 2 hours ------ Body fluids normally have no glucose. Presence of glucose indicates infection or inflammation. 1 ml BODY FLUID NO NORMAL RANGE ESTABLISHED CODE: GLFL CPT: 82945 SCHEDULE: DAILY GLUCOSE, PREGNANT RELATED 1 HOUR Results indicate glucose metabolism response to the glucola load. 1 ml SERUM COLLECTED ONE HOUR ------ 48 hours ------ LOADING (O'SULLIVAN SCREEN) 70-139 mg/dL AFTER 50 gm GLUCOSE LOAD CODE: GESC CPT: 82947 SCHEDULE: DAILY The blood glucose test may be used to: GLUCOSE, SERUM (FASTING AND RANDOM) 60-115 mg/dL ------ 48 hours ------ 1 ml SERUM Screen for both high blood glucose AGE AND GENDER DEPENDENT CODE: GLU Help diagnose diabetes CPT: 82947 SCHEDULE: DAILY Monitor glucose levels in persons with diabetes GLUCOSE TOLERANCE TEST 8HR FAST REQUIRED. COLLECT AND CRITERIA FOR DIAGNOSIS OF ------ 48 hours ------ The glucose tolerance test monitors glucose metabolism over a certain time period. -

Ionized Calcium: Analytical Challenges and Clinical Relevance
16 Review Article Page 1 of 16 Ionized calcium: analytical challenges and clinical relevance Aghilès Hamroun1, Jean-David Pekar2, Arnaud Lionet1, Amjad Ghulam3, Patrice Maboudou2, Ambroise Mercier2, Thierry Brousseau2, Guillaume Grzych3,4, François Glowacki1,5 1CHU Lille, Service de Néphrologie, F-59000, Lille, France; 2CHU Lille, Biochimie Automatisée (UF-8832), Pôle de Biologie Pathologie Génétique, F-59000, Lille, France; 3CHU Lille, Service « Hormonologie, Métabolisme, Nutrition, Oncologie », Pôle de Biologie Pathologie Génétique, Lille, France; 4INSERM, U1011-EGID, Institut Pasteur de Lille, F-59000, Lille, France; 5EA 4483-IMPECS-IMPact of Environmental Chemicals on Human Health, Research Department, 59045, Lille, France Contributions: (I) Conception and design: G Grzych, JD Pekar, A Hamroun; (II) Administrative support: G Grzych, JD Pekar, A Hamroun; (III) Provision of study materials or patients: None; (IV) Collection and assembly of data: G Grzych, JD Pekar, A Hamroun; (V) Data analysis and interpretation: G Grzych, JD Pekar, A Hamroun; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors. Correspondence to: Dr. Guillaume Grzych, MD, MSc. Laboratory of Biochemistry, Biology and Pathology Center, Lille University Hospital, Pr J. Leclercq Boulevard, 59037 Lille, France. Email: [email protected]. Abstract: Calcium is a central electrolyte involved in many physiological functions commonly measured in clinical practice. Abnormal calcium levels are frequently found, and could have serious clinical consequences. The free form of calcium, also called ionized calcium, plays a determining role in the human body. Its measurement still remains limited by technical, analytical and financial constraints. To overcome these limits, many formulas have been developed to estimate the ionized calcium value.