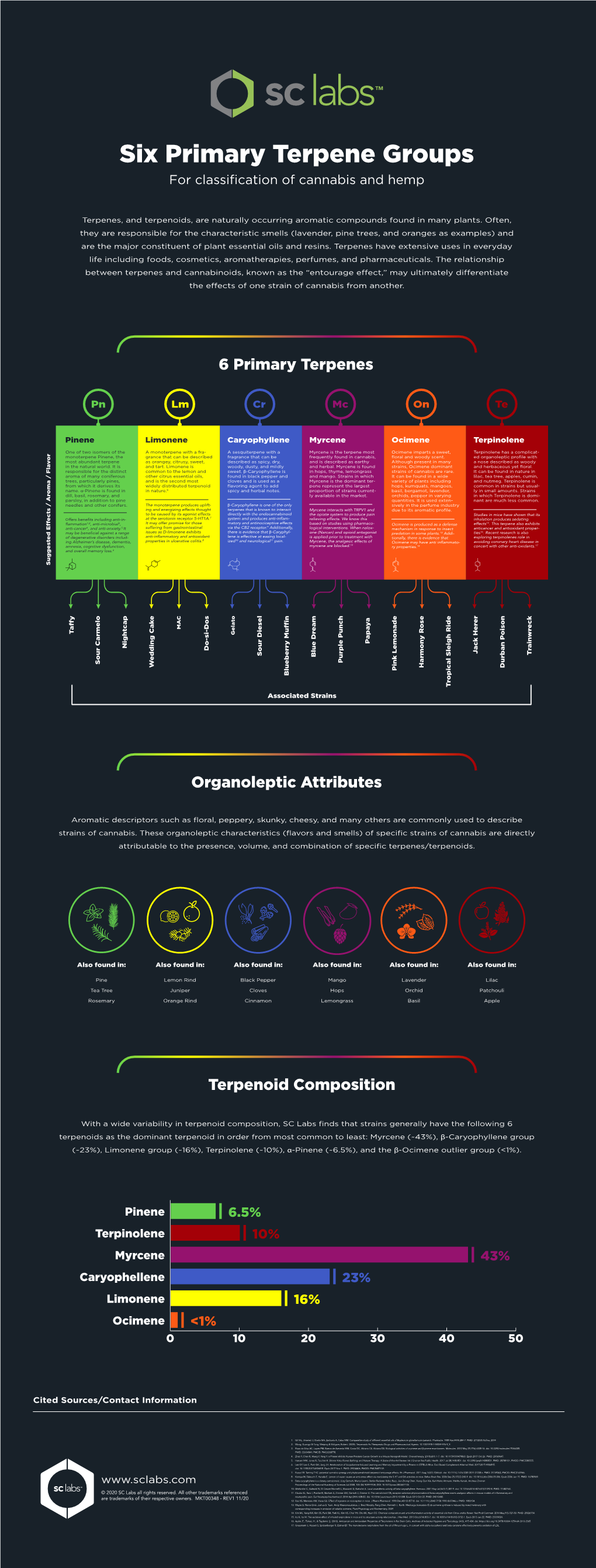
Terpenoid Composition Organoleptic Attributes 6 Primary Terpenes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Understanding and Managing the Transition Using Essential Oils Vs
MENOPAUSE: UNDERSTANDING AND MANAGING THE TRANSITION USING ESSENTIAL OILS VS. TRADITIONAL ALLOPATHIC MEDICINE by Melissa A. Clanton A thesis submitted in partial fulfillment of the requirements for the Diploma of Aromatherapy 401 Australasian College of Health Sciences Instructors: Dorene Petersen, Erica Petersen, E. Joy Bowles, Marcangelo Puccio, Janet Bennion, Judika Illes, and Julie Gatti TABLE OF CONTENTS List of Tables and Figures............................................................................ iv Acknowledgments........................................................................................ v Introduction.................................................................................................. 1 Chapter 1 – Female Reproduction 1a – The Female Reproductive System............................................. 4 1b - The Female Hormones.............................................................. 9 1c – The Menstrual Cycle and Pregnancy....................................... 12 Chapter 2 – Physiology of Menopause 2a – What is Menopause? .............................................................. 16 2b - Physiological Changes of Menopause ..................................... 20 2c – Symptoms of Menopause ....................................................... 23 Chapter 3 – Allopathic Approaches To Menopausal Symptoms 3a –Diagnosis and Common Medical Treatments........................... 27 3b – Side Effects and Risks of Hormone Replacement Therapy ...... 32 3c – Retail Cost of Common Hormone Replacement -

Biosynthesis of New Alpha-Bisabolol Derivatives Through a Synthetic Biology Approach Arthur Sarrade-Loucheur
Biosynthesis of new alpha-bisabolol derivatives through a synthetic biology approach Arthur Sarrade-Loucheur To cite this version: Arthur Sarrade-Loucheur. Biosynthesis of new alpha-bisabolol derivatives through a synthetic biology approach. Biochemistry, Molecular Biology. INSA de Toulouse, 2020. English. NNT : 2020ISAT0003. tel-02976811 HAL Id: tel-02976811 https://tel.archives-ouvertes.fr/tel-02976811 Submitted on 23 Oct 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. THÈSE En vue de l’obtention du DOCTORAT DE L’UNIVERSITÉ DE TOULOUSE Délivré par l'Institut National des Sciences Appliquées de Toulouse Présentée et soutenue par Arthur SARRADE-LOUCHEUR Le 30 juin 2020 Biosynthèse de nouveaux dérivés de l'α-bisabolol par une approche de biologie synthèse Ecole doctorale : SEVAB - Sciences Ecologiques, Vétérinaires, Agronomiques et Bioingenieries Spécialité : Ingénieries microbienne et enzymatique Unité de recherche : TBI - Toulouse Biotechnology Institute, Bio & Chemical Engineering Thèse dirigée par Gilles TRUAN et Magali REMAUD-SIMEON Jury -

DIFFERENCES in Terpenoid Levels Between Plant Species Are Known To
GENETICS OF TEP.PENES I. GENE CONTROL OF MONOTERPENE LEVELS IN PINUS MONTICOLA DOUGL. JAMES W. HANOVER Geneticist, Forestry Sciences Laboratory, lntermountain Forest and Range Experiment Station, U.S. Department of Agriculture, Moscow, Idaho * Receivedi o.v.6 1.INTRODUCTION DIFFERENCESin terpenoid levels between plant species are known to exist but little is known about their genetic bases. The terpenes have been used extensively in biochemical systematic studies in Pinus (Mirov, 1958, 1961; Williams and Bannister, 1962; Forde, 1964), Eucalyptus (Baker and Smith, 1920; Penfold and Morrison, 1927), Cup ressacee (Erdtman, 1958),andmany other plant families and genera (Alston and Turner, 1963).Thesestudies were usually based upon the assumption that variation within a species is relatively small. Although this may be valid in some cases, Bannister et al. (1962)andothers have shown that the level of terpenes can vary with geographic origin within a species. Recent data on tree-to-tree variability in the monoterpenes of Pinus ponderosa Laws. show that such variation can be relatively large (Smith, 1964). Results of similar work in our laboratory with western white pine (Pinus monticola Dougi.) also revealed substantial qualitative and quantitative variation in the cortex monoterpenes between trees. In order to provide a basis for the use of terpenes for comparative biochemical studies, an understanding of both their variability and mode of inheritance is essential. The objective of the present study is to demonstrate the degree to which levels of six monoterpene compounds are gene-controlled in western white pine. Interrelations among the terpenes and between terpenes and growth are also considered, 2.MATERIALS AND METHODS Thematerials for this study are of three types: (i) parents—as clonal lines of grafts, () F1 progeny from crosses between the ortets (parents) represented by the clones, and () S1 progeny of the parent trees. -

Advances in Azorella Glabra Wedd. Extract Research: in Vitro
molecules Article Advances in Azorella glabra Wedd. Extract Research: In Vitro Antioxidant Activity, Antiproliferative Effects on Acute Myeloid Leukemia Cells and Bioactive Compound Characterization 1, , 2,3, 1 1 Daniela Lamorte * y , Immacolata Faraone y , Ilaria Laurenzana , Stefania Trino , Daniela Russo 2,3 , Dilip K. Rai 4 , Maria Francesca Armentano 2,3 , Pellegrino Musto 5 , 6 7 2,3, , 7, Alessandro Sgambato , Luciana De Luca , Luigi Milella * z and Antonella Caivano z 1 Laboratory of Preclinical and Translational Research, Centro di Riferimento Oncologico della Basilicata (IRCCS CROB), 85028 Rionero in Vulture, Potenza, Italy; [email protected] (I.L.); [email protected] (S.T.) 2 Department of Science, University of Basilicata, V.le dell’Ateneo Lucano 10, 85028 Rionero in Vulture, Potenza, Italy; [email protected] (I.F.); [email protected] (D.R.); [email protected] (M.F.A.) 3 Spinoff BioActiPlant s.r.l., University of Basilicata, V.le dell’Ateneo Lucano 10, 85028 Rionero in Vulture, Potenza, Italy 4 Department of Food BioSciences, Teagasc Food Research Centre Ashtown, D15KN3K Dublin, Ireland; [email protected] 5 Hematology and Stem Cell Transplantation Unit, Centro di Riferimento Oncologico della Basilicata (IRCCS CROB), 85028 Rionero in Vulture, Potenza, Italy; [email protected] 6 Scientific Direction, Centro di Riferimento Oncologico della Basilicata (IRCCS CROB), 85028 Rionero in Vulture, Potenza, Italy; [email protected] 7 Unit of Clinical Pathology, Centro di Riferimento Oncologico della Basilicata (IRCCS CROB), 85028 Rionero in Vulture, Potenza, Italy; [email protected] (L.D.L.); [email protected] (A.C.) * Correspondence: [email protected] (D.L.); [email protected] (L.M.); Tel.: +39-0972-726528 (D.L.); +39-0971-205525 (L.M.) These authors contributed equally to this work. -

The Composition of Cigarette Smoke I. Solanesyl Acetate
The Composition of Cigarette Smoke I. Solanesyl Acetate Alan Rodgman and Lawrence C. Cook Research Department, R. J. Reynolds Tobaeco Company Winston-Salem, North Carolina, U.S.A. Tobacco Science, 1959, 3-20, p. 86-88, ISSN.0082-4523.pdf Solanesol, an unsaturated penta confirmation of the identity of the of solanesyl acetate from the smoke terpenoid alcohol, has been identified acid was provided by conversion of of Cigarettes B was an artifact due as a constituent of flue-cured tobacco the hydrated sodium acetate to to ester interchange occurring on the at various stages of treatment by p-pheny]phenacyl acetate (Shriner adsorbent between solanesol in the Rowland, Latimer and Giles (1956). and Fuson, 1948). These data firmly particular fraction investigated and Several solanesyl esters, namely, the establish the identity of solanesyl the ethyl acetate employed as eluent. caprate, caprylate, myristate, palmi acetate as a constituent of the ciga Mold and Booth (1957) reported tate, oleate, linoleate and linolenate, rette smoke. 130 mg. of sokmesol per 1000 ciga have also been reported in an extract Two separate lots of cigarettes of rettes smoked. We found 230 mg. of of flue-cured tobacco (Rowland and the same blend were smoked during this material per 1000 Cigarettes A Latimer, 1959). Recently, Rowland this investigation. One, Cigarettes A, and 370 mg. per 1000 Cigarettes B; (1959) has identified solanesyl ace consisting of 20,000 cigarettes, gave values approximately two or three tate in an extract of flue-cured to 0.35 g. (0.0025 per cent of tobacco times that of Mold and Booth. -

Determination of Terpenoid Content in Pine by Organic Solvent Extraction and Fast-Gc Analysis
ORIGINAL RESEARCH published: 25 January 2016 doi: 10.3389/fenrg.2016.00002 Determination of Terpenoid Content in Pine by Organic Solvent Extraction and Fast-GC Analysis Anne E. Harman-Ware1* , Robert Sykes1 , Gary F. Peter2 and Mark Davis1 1 National Bioenergy Center, National Renewable Energy Laboratory, Golden, CO, USA, 2 School of Forest Resources and Conservation, University of Florida, Gainesville, FL, USA Terpenoids, naturally occurring compounds derived from isoprene units present in pine oleoresin, are a valuable source of chemicals used in solvents, fragrances, flavors, and have shown potential use as a biofuel. This paper describes a method to extract and analyze the terpenoids present in loblolly pine saplings and pine lighter wood. Various extraction solvents were tested over different times and temperatures. Samples were analyzed by pyrolysis-molecular beam mass spectrometry before and after extractions to monitor the extraction efficiency. The pyrolysis studies indicated that the optimal extraction method used a 1:1 hexane/acetone solvent system at 22°C for 1 h. Extracts from the hexane/acetone experiments were analyzed using a low thermal mass modular accelerated column heater for fast-GC/FID analysis. The most abundant terpenoids from Edited by: the pine samples were quantified, using standard curves, and included the monoter- Subba Rao Chaganti, University of Windsor, Canada penes, α- and β-pinene, camphene, and δ-carene. Sesquiterpenes analyzed included Reviewed by: caryophyllene, humulene, and α-bisabolene. Diterpenoid resin acids were quantified in Yu-Shen Cheng, derivatized extractions, including pimaric, isopimaric, levopimaric, palustric, dehydroabi- National Yunlin University of Science and Technology, Taiwan etic, abietic, and neoabietic acids. -

Solanesol Biosynthesis in Plants
Review Solanesol Biosynthesis in Plants Ning Yan *, Yanhua Liu, Hongbo Zhang, Yongmei Du, Xinmin Liu and Zhongfeng Zhang Tobacco Research Institute of Chinese Academy of Agricultural Sciences, Qingdao 266101, China; [email protected] (Y.L.); [email protected] (H.Z.); [email protected] (Y.D.); [email protected] (X.L.); [email protected] (Z.Z.) * Correspondence: [email protected]; Tel.: +86-532-8870-1035 Academic Editor: Derek J. McPhee Received: 1 March 2017; Accepted: 22 March 2017; Published: 23 March 2017 Abstract: Solanesol is a non-cyclic terpene alcohol composed of nine isoprene units that mainly accumulates in solanaceous plants. Solanesol plays an important role in the interactions between plants and environmental factors such as pathogen infections and moderate-to-high temperatures. Additionally, it is a key intermediate for the pharmaceutical synthesis of ubiquinone-based drugs such as coenzyme Q10 and vitamin K2, and anti-cancer agent synergizers such as N-solanesyl- N,N′-bis(3,4-dimethoxybenzyl) ethylenediamine (SDB). In plants, solanesol is formed by the 2-C-methyl-D-erythritol 4-phosphate (MEP) pathway within plastids. Solanesol’s biosynthetic pathway involves the generation of C5 precursors, followed by the generation of direct precursors, and then the biosynthesis and modification of terpenoids; the first two stages of this pathway are well understood. Based on the current understanding of solanesol biosynthesis, we here review the key enzymes involved, including 1-deoxy-D-xylulose 5-phosphate synthase (DXS), 1-deoxy-D- xylulose 5-phosphate reductoisomerase (DXR), isopentenyl diphosphate isomerase (IPI), geranyl geranyl diphosphate synthase (GGPPS), and solanesyl diphosphate synthase (SPS), as well as their biological functions. -

Medically Useful Plant Terpenoids: Biosynthesis, Occurrence, and Mechanism of Action
molecules Review Medically Useful Plant Terpenoids: Biosynthesis, Occurrence, and Mechanism of Action Matthew E. Bergman 1 , Benjamin Davis 1 and Michael A. Phillips 1,2,* 1 Department of Cellular and Systems Biology, University of Toronto, Toronto, ON M5S 3G5, Canada; [email protected] (M.E.B.); [email protected] (B.D.) 2 Department of Biology, University of Toronto–Mississauga, Mississauga, ON L5L 1C6, Canada * Correspondence: [email protected]; Tel.: +1-905-569-4848 Academic Editors: Ewa Swiezewska, Liliana Surmacz and Bernhard Loll Received: 3 October 2019; Accepted: 30 October 2019; Published: 1 November 2019 Abstract: Specialized plant terpenoids have found fortuitous uses in medicine due to their evolutionary and biochemical selection for biological activity in animals. However, these highly functionalized natural products are produced through complex biosynthetic pathways for which we have a complete understanding in only a few cases. Here we review some of the most effective and promising plant terpenoids that are currently used in medicine and medical research and provide updates on their biosynthesis, natural occurrence, and mechanism of action in the body. This includes pharmacologically useful plastidic terpenoids such as p-menthane monoterpenoids, cannabinoids, paclitaxel (taxol®), and ingenol mebutate which are derived from the 2-C-methyl-d-erythritol-4-phosphate (MEP) pathway, as well as cytosolic terpenoids such as thapsigargin and artemisinin produced through the mevalonate (MVA) pathway. We further provide a review of the MEP and MVA precursor pathways which supply the carbon skeletons for the downstream transformations yielding these medically significant natural products. Keywords: isoprenoids; plant natural products; terpenoid biosynthesis; medicinal plants; terpene synthases; cytochrome P450s 1. -

Biosynthetic Origin of Complex Terpenoid Mixtures by Multiproduct Enzymes, Metal Cofactors, and Substrate Isomers
Natural Products Chemistry & Research Review Article Biosynthetic Origin of Complex Terpenoid Mixtures by Multiproduct Enzymes, Metal Cofactors, and Substrate Isomers Vattekkatte A, Boland W * Department of Bioorganic Chemistry, Max Planck Institute for Chemical Ecology, Beutenberg Campus, Hans-Knöll-Strasse 8, D-07745 Jena, Germany ABSTRACT Terpenoids form a substantial portion of chemical diversity in nature. The enormous terpenoid diversity of more than 80,000 compounds is supported by the multisubstrate and multiproduct nature of certain enzymes from the various terpene synthases and terpene cyclases. These highly versatile enzymes are not only able to accept multiple substrates in their active site, but also simultaneously catalyze multiple reactions to the resultant multiple products. Interestingly, apart from the substrates and catalytic mechanisms, multiple regulation factors are able to alter the product profile of multiproduct terpene synthases. Simple variations in cellular conditions by changes in metal cofactors, assay pH, temperature and substrate geometry lead to significant shifts in product profiles. Switch in substrate stereochemistry for multiproduct terpene synthases in some case shows enhanced biocatalysis and in others initiates even a novel cyclization cascade. Hence, organisms can get access to a greater chemodiversity and avoid the expensive process of developing new biocatalysts just by simple changes in the cellular environment. This possibility of modulating chemical diversity provides immobile plants in the same generation access to an enhanced chemical arsenal for defense and communication by simply altering cofactors, pH level, and temperature and substrate geometry. Keywords: Terpenoids; Biocatalysis; Polymers; Substrate isomers; Catalysis INTRODUCTION and waxy cuticles acts as sunscreen, plant polymers like lignin Plants being immobile organisms do not have the ability to and rubber provide support and wound healing. -

Preparation of Terpenoid-Invasomes with Selective Activity Against S
biomedicines Article Preparation of Terpenoid-Invasomes with Selective Activity against S. aureus and Characterization by Cryo Transmission Electron Microscopy Bernhard P. Kaltschmidt 1, Inga Ennen 1, Johannes F. W. Greiner 2 , Robin Dietsch 3, 3 2,4 2, 1, , Anant Patel , Barbara Kaltschmidt , Christian Kaltschmidt y and Andreas Hütten * y 1 Thin Films & Physics of Nanostructures, Bielefeld University, Universitätsstrasse 25, 33615 Bielefeld, Germany; [email protected] (B.P.K.); [email protected] (I.E.) 2 Department of Cell Biology, Bielefeld University, Universitätsstrasse 25, 33615 Bielefeld, Germany; [email protected] (J.F.W.G.); [email protected] (B.K.); [email protected] (C.K.) 3 Fermentation and Formulation of Biologicals and Chemicals, Bielefeld University of Applied Sciences, Interaktion 1, 33619 Bielefeld, Germany; [email protected] (R.D.); [email protected] (A.P.) 4 Molecular Neurobiology, Bielefeld University, Universitätsstrasse 25, 33615 Bielefeld, Germany * Correspondence: [email protected]; Tel.: +49-521-106-5418 These authors contributed equally to this work. y Received: 7 April 2020; Accepted: 30 April 2020; Published: 1 May 2020 Abstract: Terpenoids are natural plant-derived products that are applied to treat a broad range of human diseases, such as airway infections and inflammation. However, pharmaceutical applications of terpenoids against bacterial infection remain challenging due to their poor water solubility. Here, we produce invasomes encapsulating thymol, menthol, camphor and 1,8-cineol, characterize them via cryo transmission electron microscopy and assess their bactericidal properties. While control- and cineol-invasomes are similarly distributed between unilamellar and bilamellar vesicles, a shift towards unilamellar invasomes is observable after encapsulation of thymol, menthol or camphor. -

Terpene and Terpenoid Emissions and Secondary Organic Aerosol Production
Michigan Technological University Digital Commons @ Michigan Tech Dissertations, Master's Theses and Master's Dissertations, Master's Theses and Master's Reports - Open Reports 2013 TERPENE AND TERPENOID EMISSIONS AND SECONDARY ORGANIC AEROSOL PRODUCTION Rosa M. Flores Michigan Technological University Follow this and additional works at: https://digitalcommons.mtu.edu/etds Part of the Atmospheric Sciences Commons, and the Environmental Engineering Commons Copyright 2013 Rosa M. Flores Recommended Citation Flores, Rosa M., "TERPENE AND TERPENOID EMISSIONS AND SECONDARY ORGANIC AEROSOL PRODUCTION", Dissertation, Michigan Technological University, 2013. https://doi.org/10.37099/mtu.dc.etds/818 Follow this and additional works at: https://digitalcommons.mtu.edu/etds Part of the Atmospheric Sciences Commons, and the Environmental Engineering Commons TERPENE AND TERPENOID EMISSIONS AND SECONDARY ORGANIC AEROSOL PRODUCTION By Rosa M. Flores A DISSERTATION Submitted in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY In Environmental Engineering MICHIGAN TECHNOLOGICAL UNIVERSITY 2013 © Rosa M. Flores This dissertation has been approved in partial fulfillment of the requirements for the Degree of DOCTOR OF PHILOSOPHY in Environmental Engineering. Department of Civil and Environmental Engineering Dissertation Advisor: Paul V. Doskey Committee Member : Chandrashekhar P. Joshi Committee Member : Claudio Mazzoleni Committee Member : Lynn Mazzoleni Committee Member : Judith Perlinger Department Chair: David Hand To dad -

Dimethylallyl Pyrophosphate Is Not the Committed Precursor of Isopentenyl Pyrophosphate During Terpenoid Biosynthesis from 1-Deoxyxylulose in Higher Plants
Proc. Natl. Acad. Sci. USA Vol. 96, pp. 1309–1314, February 1999 Biochemistry Dimethylallyl pyrophosphate is not the committed precursor of isopentenyl pyrophosphate during terpenoid biosynthesis from 1-deoxyxylulose in higher plants DUILIO ARIGONI*†,WOLFGANG EISENREICH‡,CHRISTOPH LATZEL§,SILVIA SAGNER§,TANJA RADYKEWICZ‡, MEINHART H. ZENK§, AND ADELBERT BACHER‡ *Laboratorium fu¨r Organische Chemie, Eidgeno¨ssische Technische Hochschule, Universita¨tsstrasse16, CH-8092 Zurich, Switzerland; §Lehrstuhl fu¨r Pharmazeutische Biologie, Universita¨t Mu¨nchen, Karlstrasse 29, D-80333 Munich, Germany; and ‡Lehrstuhl fu¨r Organische Chemie und Biochemie, Technische Universita¨t Mu¨nchen, Lichtenbergstrasse 4, D-85747 Garching, Germany Contributed by Duilio Arigoni, December 18, 1998 ABSTRACT Cell cultures of Catharanthus roseus were way to isopentenyl pyrophosphate (IPP) (5) and dimethylallyl supplied with [2-13C,3-2H]-deoxyxylulose or [2-13C,4-2H]1- pyrophosphate (DMAPP) (6) have been identified. deoxyxylulose. Lutein and chlorophylls were isolated from the Phytol (7, Fig. 2), b-carotene, and lutein (8) are biosynthe- cell mass, and hydrolysis of the chlorophyll mixtures afforded sized in Catharanthus roseus from DMAPP and IPP stemming phytol. Isotope labeling patterns of phytol and lutein were largely, if not exclusively, from deoxyxylulose (9). Specifically, determined by 2H NMR and 1H,2H-decoupled 13C NMR. From DMAPP serves as a starter unit, which is converted into the the data it must be concluded that the deuterium atom in common precursor geranylgeranyl pyrophosphate by sequen- position 3 of deoxyxylulose was incorporated into both iso- tial reactions with three IPP units. To obtain information on pentenyl pyrophosphate (IPP) and dimethylallyl pyrophos- the nature and sequence of the unknown steps that lead from 13 phate with a rate of 75% (with respect to the internal C 1-deoxyxylulose to the two C5 building blocks of terpene label).