BSAI Squids Is Updated with 2005 and Partial 2006 Data
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Review of Southern Ocean Squids Using Nets and Beaks
Marine Biodiversity (2020) 50:98 https://doi.org/10.1007/s12526-020-01113-4 REVIEW A review of Southern Ocean squids using nets and beaks Yves Cherel1 Received: 31 May 2020 /Revised: 31 August 2020 /Accepted: 3 September 2020 # Senckenberg Gesellschaft für Naturforschung 2020 Abstract This review presents an innovative approach to investigate the teuthofauna from the Southern Ocean by combining two com- plementary data sets, the literature on cephalopod taxonomy and biogeography, together with predator dietary investigations. Sixty squids were recorded south of the Subtropical Front, including one circumpolar Antarctic (Psychroteuthis glacialis Thiele, 1920), 13 circumpolar Southern Ocean, 20 circumpolar subantarctic, eight regional subantarctic, and 12 occasional subantarctic species. A critical evaluation removed five species from the list, and one species has an unknown taxonomic status. The 42 Southern Ocean squids belong to three large taxonomic units, bathyteuthoids (n = 1 species), myopsids (n =1),andoegopsids (n = 40). A high level of endemism (21 species, 50%, all oegopsids) characterizes the Southern Ocean teuthofauna. Seventeen families of oegopsids are represented, with three dominating families, onychoteuthids (seven species, five endemics), ommastrephids (six species, three endemics), and cranchiids (five species, three endemics). Recent improvements in beak identification and taxonomy allowed making new correspondence between beak and species names, such as Galiteuthis suhmi (Hoyle 1886), Liguriella podophtalma Issel, 1908, and the recently described Taonius notalia Evans, in prep. Gonatus phoebetriae beaks were synonymized with those of Gonatopsis octopedatus Sasaki, 1920, thus increasing significantly the number of records and detailing the circumpolar distribution of this rarely caught Southern Ocean squid. The review extends considerably the number of species, including endemics, recorded from the Southern Ocean, but it also highlights that the corresponding species to two well-described beaks (Moroteuthopsis sp. -

Technical Report No. 447 1974 •
FISHERIES RESEARCH BOARD OF CANADA TECHNICAL REPORT NO. 447 1974 • ... FISHERIES RESEARCH BOARD OF CANADA Technical Reports FRB Technical Reports are research documents that are of sufficient importance to be preserved, but which ·for some reason are not appropriate for primary scientific publication. No restriction is placed on subject matter and the series should reflect the broad research interests of FRB. These Reports can be cited in publications, but care should be taken to indicate their manuscript status. Some of the material in these Reports will eventually appear in the primary scientific literature. Inquiries concerning any particular Report should be directed to the issuing FRB establishment which is indicated on the title page. FISHERIES AND MARINE SERVICE TECHNICAL REPORT NO. 44 7 THE SQUID OF BRITISH COLUMBIA AS A POTENTIAL FISHERY RESOURCE - A PRELIMINARY REPORT by S.A. Macfarlane and M. Yamamoto Fisheries and Marine Service Vancouver Laboratory Vancouver, B.C. TABLE OF CONTENTS Page No. I. INTRODUCTION 1 II. BIOLOGICAL ASPECTS 3 III. COMMERCIAL ASPECTS 8 A. Fishing Methods 8 B. International Squid Fisher,y 15 c. Status of Squid in British Co1uabia 19 IV. NUTRITIONAL ASPECTS 27 v. PROCESSING 28 VI. DISCUSSION 30 VII. ACKNOWLEDGMENTS 32 VIII. REFERENCES 33 1. I. INTRODUCTION Available catch statistics from 1965 through 1971 indicate • that world-wide landings of squid totalled roughly 700,000 metric tons annually. An additional 100,000 metric tons of cuttlefish and about 160,000 metric tons of octopus were also landed annually. Apart from the well-established squid fishery in the Monterey area of California and the relatively minor inshore squid fishery off Newfoundland, the North American fishing industry has tended to ignore the possibility of further exploitation and utilization of this resource. -

Forage Fish Management Plan
Oregon Forage Fish Management Plan November 19, 2016 Oregon Department of Fish and Wildlife Marine Resources Program 2040 SE Marine Science Drive Newport, OR 97365 (541) 867-4741 http://www.dfw.state.or.us/MRP/ Oregon Department of Fish & Wildlife 1 Table of Contents Executive Summary ....................................................................................................................................... 4 Introduction .................................................................................................................................................. 6 Purpose and Need ..................................................................................................................................... 6 Federal action to protect Forage Fish (2016)............................................................................................ 7 The Oregon Marine Fisheries Management Plan Framework .................................................................. 7 Relationship to Other State Policies ......................................................................................................... 7 Public Process Developing this Plan .......................................................................................................... 8 How this Document is Organized .............................................................................................................. 8 A. Resource Analysis .................................................................................................................................... -

ICES Marine Science Symposia
ICES mar. Sei. Symp., 199: 459-467. 1995 Genetic differentiation in Berryteuthis magister from the North Pacific O. N. Katugin Katugin, O. N. 1995. Genetic differentiation in Berryteuthis magister from the North Pacific. - ICES mar. Sei. Symp., 199: 459-467. Berryteuthis magister is a widespread quasibenthic commercial squid from the North Pacific. Intraspecific genetic differentiation was determined by allozyme electrophore tic analysis. Eighteen sample lots (2100 individuals) from geographically separated North Pacific regions were subjected to allozyme electrophoretic analysis using a total of 14 enzymes and unidentified ganglion protein spectra with polymorphic zones. Four loci with variant allele frequencies greater than 0.05 were found to be useful for population studies. No significant violations of the Hardy-Weinberg equilibrium were found at any loci in the samples. There was no evidence of genetic differences between sexes. Analysis of genetic differentiation using Wright’s F-statistics, cluster analysis of genetic distances, and contingency chi-square analysis suggested that there are popu lation differences between squids from the three major geographical localities: the Sea of Japan, the Kurile-Komandor region, and the Gulf of Alaska. Genetic divergence between squid from the Kurile-Komandor part of the species range probably reflects subpopulation differentiation of local stocks from successive generations. O. N. Katugin: Pacific Research Institute o f Fisheries and Oceanography (TINRO), Vladivostok, 690 600, Russia [tel: (+7) 4232 25 7790, fax: (+7) 4232 25 7783], been investigated electrophoretically. This family is con Introduction sidered to be the most abundant group of cephalopods in During the last two decades biochemical genetic tech the subarctic waters of the Pacific Ocean where it pre niques based on electrophoretic separation of multiple sumably originated and diverged (Nesis, 1973). -

December 20, 2007
BEFORE THE SECRETARY OF COMMERCE PETITION TO LIST THE RIBBON SEAL (HISTRIOPHOCA FASCIATA) AS A THREATENED OR ENDANGERED SPECIES UNDER THE ENDANGERED SPECIES ACT © G. CARLETON RAY CENTER FOR BIOLOGICAL DIVERSITY DECEMBER 20, 2007 Notice of Petition____________________________________________________ Carlos M. Gutierrez Secretary of Commerce U.S. Department of Commerce 1401 Constitution Avenue, N.W., Room 5516 Washington, D.C. 20230 Dr. William Hogarth Assistant Administrator for Fisheries National Oceanographic and Atmospheric Administration 1315 East-West Highway Silver Springs, MD 20910 PETITIONER The Center for Biological Diversity 1095 Market Street, Suite 511 San Francisco, CA 94103 ph: (415) 436-9682 ext 301 fax: (415) 436-9683 __________________________ Date: this 20th day of December, 2007 Shaye Wolf, Ph.D. Martha Palomino Tovar, Ph.D. Candidate Brendan Cummings Center for Biological Diversity Pursuant to Section 4(b) of the Endangered Species Act (“ESA”), 16 U.S.C. §1533(b), Section 553(3) of the Administrative Procedures Act, 5 U.S.C. § 553(e), and 50 C.F.R. §424.14(a), the Center for Biological Diversity (“Petitioner”) hereby petitions the Secretary of Commerce, through the National Marine Fisheries Service (“NMFS”), to list the ribbon seal (Histriophoca fasciata) as a threatened or endangered species and to designate critical habitat to ensure its survival and recovery. The Center for Biological Diversity (“Center”) is a non-profit, public interest environmental organization dedicated to the protection of native species and their habitats through science, policy, and environmental law. The Center has over 40,000 members in Alaska and throughout the United States. The Center and its members are concerned with the conservation of endangered species, including the ribbon seal, and the effective implementation of the ESA. -

Translation 3204
4 of 6 I' rÉ:1°.r - - - Ï''.ec.n::::,- - — TRANSLATION 3204 and Van, else--- de ,-0,- SERIES NO(S) ^4p €'`°°'°^^`m`^' TRANSLATION 3204 5 of 6 serceaesoe^nee SERIES NO.(S) serv,- i°- I' ann., Canada ° '° TRANSLATION 3204 6 of 6 SERIES NO(S) • =,-""r I FISHERIES AND MARINE SERVICE ARCHIVE:3 Translation Series No. 3204 Multidisciplinary investigations of the continental slope in the Gulf of Alaska area by Z.A. Filatova (ed.) Original title: Kompleksnyye issledovaniya materikovogo sklona v raione Zaliva Alyaska From: Trudy Instituta okeanologii im. P.P. ShirshoV (Publications of the P.P. Shirshov Oceanpgraphy Institute), 91 : 1-260, 1973 Translated by the Translation Bureau(HGC) Multilingual Services Division Department of the Secretary of State of Canada Department of the Environment Fisheries and Marine Service Pacific Biological Station Nanaimo, B.C. 1974 ; 494 pages typescriPt "DEPARTMENT OF THE SECRETARY OF STATE SECRÉTARIAT D'ÉTAT TRANSLATION BUREAU BUREAU DES TRADUCTIONS MULTILINGUAL SERVICES DIVISION DES SERVICES DIVISION MULTILINGUES ceÔ 'TRANSLATED FROM - TRADUCTION DE INTO - EN Russian English Ain HOR - AUTEUR Z. A. Filatova (ed.) ri TL E IN ENGLISH - TITRE ANGLAIS Multidisciplinary investigations of the continental slope in the Gulf of Aâaska ares TI TLE IN FORE I GN LANGuAGE (TRANS LI TERA TE FOREIGN CHARACTERS) TITRE EN LANGUE ÉTRANGÈRE (TRANSCRIRE EN CARACTÈRES ROMAINS) Kompleksnyye issledovaniya materikovogo sklona v raione Zaliva Alyaska. REFERENCE IN FOREI GN LANGUAGE (NAME: OF BOOK OR PUBLICATION) IN FULL. TRANSLI TERATE FOREIGN CHARACTERS, RÉFÉRENCE EN LANGUE ÉTRANGÈRE (NOM DU LIVRE OU PUBLICATION), AU COMPLET, TRANSCRIRE EN CARACTÈRES ROMAINS. Trudy Instituta okeanologii im. P.P. -

An Illustrated Key to the Families of the Order
CLYDE F. E. ROP An Illustrated RICHARD E. YOl and GILBERT L. VC Key to the Families of the Order Teuthoidea Cephalopoda) SMITHSONIAN CONTRIBUTIONS TO ZOOLOGY • 1969 NUMBER 13 SMITHSONIAN CONTRIBUTIONS TO ZOOLOGY NUMBER 13 Clyde F. E. Roper, An Illustrated Key 5K?Z" to the Families of the Order Teuthoidea (Cephalopoda) SMITHSONIAN INSTITUTION PRESS CITY OF WASHINGTON 1969 SERIAL PUBLICATIONS OF THE SMITHSONIAN INSTITUTION The emphasis upon publications as a means of diffusing knowledge was expressed by the first Secretary of the Smithsonian Institution. In his formal plan for the Institution, Joseph Henry articulated a program that included the following statement: "It is proposed to publish a series of reports, giving an account of the new discoveries in science, and of the changes made from year to year in all branches of knowledge not strictly professional." This keynote of basic research has been adhered to over the years in the issuance of thousands of titles in serial publications under the Smithsonian imprint, commencing with Smithsonian Contributions to Knowledge in 1848 and continuing with the following active series: Smithsonian Annals of Flight Smithsonian Contributions to Anthropology Smithsonian Contributions to Astrophysics Smithsonian Contributions to Botany Smithsonian Contributions to the Earth Sciences Smithsonian Contributions to Paleobiology Smithsonian Contributions to Zoology Smithsonian Studies in History and Technology In these series, the Institution publishes original articles and monographs dealing with the research and collections of its several museums and offices and of professional colleagues at other institutions of learning. These papers report newly acquired facts, synoptic interpretations of data, or original theory in specialized fields. -
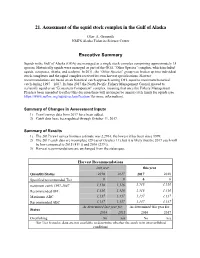
Assessment of the Squid Stock Complex in the Gulf of Alaska
21. Assessment of the squid stock complex in the Gulf of Alaska Olav A. Ormseth NMFS Alaska Fisheries Science Center Executive Summary Squids in the Gulf of Alaska (GOA) are managed as a single stock complex comprising approximately 15 species. Historically squids were managed as part of the GOA “Other Species” complex, which included squids, octopuses, sharks, and sculpins. In 2011, the “Other Species” group was broken up into individual stock complexes and the squid complex received its own harvest specifications. Harvest recommendations are based on an historical catch approach setting OFL equal to maximum historical catch during 1997 – 2007. In June 2017 the North Pacific Fishery Management Council moved to reclassify squid as an “Ecosystem Component” complex, meaning that once the Fishery Management Plan has been amended to reflect this decision there will no longer be annual catch limits for squids (see https://www.npfmc.org/squid-reclassification/ for more information). Summary of Changes in Assessment Inputs 1) Trawl survey data from 2017 have been added. 2) Catch data have been updated through October 11, 2017. Summary of Results 1) The 2017 trawl survey biomass estimate was 2,296 t, the lowest it has been since 1999. 2) The 2017 catch data are incomplete (29 t as of October 11), but it is likely that the 2017 catch will be low compared to 2015 (411 t) and 2016 (239 t). 3) Harvest recommendations are unchanged from the status quo. Harvest Recommendations last year this year Quantity/Status 2016 2017 2017 2018 Specified/recommended -

Translation 3272
ZUCHIVES FISHERIES AND MARINE SERVICE Translation Series No. 3272 Taxonomy, phylogeny and evolution of squids of the family Conatidae (Cephalopoda) by K.N. Nesis Original title: Sistema, filogeniya i evolyutsiya karmarov semeistva Gonatidae (Cephalopoda) From: Zoi31. • zh. 52(11) : 1626-1638, 1973 Translated by the Translation Bureau(TTI) Multilingual Services Division Department of the Secretary of State of Canada Department-of the Environment Fisheries and Marine Service . Biological Station ' St. John's, Nfld. , 1974 26 pages typescript DEkARTMENT OF THE SECRETARY OF STATE SECRÉTARIAT D'ÉTAT TRANSLATION BUREAU BUREAU DES TRADUCTIONS MULTILINGUAL SERVICES iktie DIVISION DES SERVICES CANADA DIVISION MULTILINGUES fid>0 7c2 Jea TRANSLATED FROM — TRADUCTION DE INTO — EN Russian English AUTHOR — AUTEUR K.N. Nesis TITLE IN ENGLISH — TITRE ANGLAIS Taxonomy, phylogeny and evolution of squids of the family Gonatidae (Cephalopoda) TITLE IN FOREIGN LANGUAGE (TRANSLITERATE FOREIGN CHARACTERS) TITRE EN LANGUE eTRANGÉRE (TRANSCRIRE EN CARACTÈRES ROMAINS) Sistema, filogeniya i evolyutsiya kal'marov semeistva Gonatidae (Cephalopoda) REFERENCE IN FOREIGN LANGUAGE (NAME OF BOOK OR PUBLICATION) IN FULL. TRANSLITERATE FOREIGN CHARACTERS. RÉFeRENCE EN LANGUE ÉTRANGÉRE (NOM DU LIVRE OU PUBLICATION), AU COMPLET, TRANSCRIRE EN CARACTÈRES ROMAINS. Zoologicheskii Zhurnal REFERENCE IN ENGLISH — ReFeRENCE EN ANGLAIS Zoological Journal PUBLISHER.- eDITEUR PAGE NUMBERS IN ORIGINAL DATE OF PUBLICATION NUMeROS DES PAGES DANS DATE DE PUBLICATION L'ORIGINAL Institute of Oceanology, USSR of Sciences YEAR ISSUE NO. Academy VOLUME 1626-1638 PLACE OF PUBLICATION ANNeE NUMeR0 NUMBER OF TYPED PAGES LIEU DE PUBLICATION NOMBRE DE PAGES DACTYLOGRAPHIÉES Moscow 1973 LII 11 26 REQUESTING DEPARTMENT TRANSLATION BUREAU NO. 676625 MINISTÈRE-CLIENT EnVirOTIMBIlt NOTRE DOSSIER NC) BRANCH OR DIVISION Fisheries Service TRANSLATOR (INITIA LS) T.Th. -

2011 GOA Squids
21. Gulf of Alaska squids Olav A. Ormseth NMFS Alaska Fisheries Science Center Executive Summary In 2010, the North Pacific Fishery Management Council passed amendment 87 to the GOA Fishery Management Plan, which separated the Other Species complex into its constituent species groups. Thus, GOA squids are now managed as an independent complex with its own harvest specifications. Because the GOA bottom trawl survey is the chief source of data for this assessment and is a biennial survey, full assessments are performed only in odd years. Summary of Changes Changes in the input data: 1. Total catch and retention data for GOA squids has been updated with complete 2010 and partial 2011 data. 2. Biomass estimates from the 2011 GOA bottom trawl survey have been added. 3. An appendix containing data regarding non-commercial catches of squid has been added to the report. Summary of Results Because reliable estimates of squid biomass and natural mortality rate do not exist, we recommend using a modified Tier 6 approach setting OFL equal to maximum historical catch and ABC equal to 0.75 * OFL using the years 1997 - 2007 as a baseline . last year this year Quantity/Status 2011 2012 2012 2013 M (natural mortality) n/a n/a n/a n/a Specified/recommended Tier 6 6 6 6 Biomass n/a n/a n/a n/a average historical catch 1997-2007 272 272 272 272 maximum historical catch 1997-2007 1,530 1,530 1,530 1,530 Recommended OFL (max. hist. catch; t) 1,530 1,530 1,530 1,530 Recommended ABC (0.75*OFL; t) 1,148 1,148 1,148 1,148 As determined last As determined this Status year for: year for: 2009 2010 2010 2011 Overfishing No n/a No n/a Overfished n/a n/a n/a n/a Approaching overfished n/a n/a n/a n/a (for Tier 6 stocks, data are not available to determine whether the stock is in an overfished condition) Introduction Description, scientific names, and general distribution Squids (order Teuthoidea) are cephalopod molluscs which are related to octopus. -

SYSTEMATICS of the CEPHALOPOD FAMILY GONATIDAE from the SOUTHEASTERN BERING SEA RECOMMENDED: APPROVED: F — > Program Head V
Systematics of the Cephalopod family Gonatidae from the southeastern Bering Sea Item Type Thesis Authors Bublitz, Christopher G. Download date 06/10/2021 03:42:26 Link to Item http://hdl.handle.net/11122/5209 SYSTEMATICS OF THE CEPHALOPOD FAMILY GONATIDAE FROM THE SOUTHEASTERN BERING SEA RECOMMENDED: f — > Programin i Head. APPROVED: Vice Chancellor ror Research and Advanced Study Date (J SYSTEMATICS OF THE CEPHALOPOD FAMILY GONATIDAE FROM THE SOUTHEASTERN BERING SEA A THESIS Presented to the Faculty of the University of Alaska in Partial Fulfillment of the Requirements for the Degree of MASTER OF SCIENCE By Qu Christopher G. Bublitz 4- o Fairbanks, Alaska '3 < o May 1981 H ABSTRACT The systematic relationships within the cephalopod family Gonatidae were examined utilizing specimens collected from the southeastern Bering Sea. Ten species; Gonatus onyx, Gonatus madokai, Gonatus tinvo3 Gonatus bewyi, Gonatus pyvos3 Gonatus sp., Gonatus type A, BevvyteuthLs magistev3 Berryteuthis anonychus3 and Gonatopsis bovealis; were identified. Included in these are the identification of a probable new species, Gonatus sp., the verification of a questionable species, Gonatus tinro, and the classification of previously described but unclassified developmental stages. Morphometric characters were used for a retrogression analysis of each species' development. The developmental stages for those species found in the study area are described and illustrated in detail. The taxonomic and morphometric characters of the species are compared. Two closely allied species, Gonatus bevvyi and Gonatus sp. are compared and contrasted. A brief an§J.ysis of the growth and development of the genus Gonatus is also given. ACKNOWLEDGEMENT S I gratefully acknowledge the assistance and encouragement given me by a large number of people, both in the United States and Japan. -

Appendix E Marine Mammal Density Report
Appendix E Marine Mammal Density Report GULF OF ALASKA NAVY TRAINING ACTIVITIES EIS/OEIS FINAL (MARCH 2011) TABLE OF CONTENTS E MARINE MAMMAL DENSITY AND DEPTH DISTRIBUTION .................................... E-1 E.1 BACKGROUND AND OVERVIEW .............................................................................................. E-1 E.1.1 DENSITY ................................................................................................................................. E-1 E.1.2 DEPTH DISTRIBUTION ......................................................................................................... E-6 E.1.3 DENSITY AND DEPTH DISTRIBUTION COMBINED ....................................................... E-6 E.2 MYSTICETES ............................................................................................................................ E-7 E.2.1 BLUE WHALE, BALAENOPTERA MUSCULUS ................................................................... E-7 E.2.2 FIN WHALE, BALAENOPTERA PHYSALUS ......................................................................... E-8 E.2.3 SEI WHALE, BALAENOPTERA BOREALIS........................................................................... E-8 E.2.4 MINKE WHALE, BALAENOPTERA ACUTOROSTRATA ..................................................... E-8 E.2.5 HUMPBACK WHALE, MEGAPTERA NOVAEANGLIAE ..................................................... E-9 E.2.6 NORTH PACIFIC RIGHT WHALE, EUBALAENA JAPONICA ............................................ E-9 E.2.7 GRAY WHALE, ESCHRICHTIUS