Translation 3272
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Review of Southern Ocean Squids Using Nets and Beaks
Marine Biodiversity (2020) 50:98 https://doi.org/10.1007/s12526-020-01113-4 REVIEW A review of Southern Ocean squids using nets and beaks Yves Cherel1 Received: 31 May 2020 /Revised: 31 August 2020 /Accepted: 3 September 2020 # Senckenberg Gesellschaft für Naturforschung 2020 Abstract This review presents an innovative approach to investigate the teuthofauna from the Southern Ocean by combining two com- plementary data sets, the literature on cephalopod taxonomy and biogeography, together with predator dietary investigations. Sixty squids were recorded south of the Subtropical Front, including one circumpolar Antarctic (Psychroteuthis glacialis Thiele, 1920), 13 circumpolar Southern Ocean, 20 circumpolar subantarctic, eight regional subantarctic, and 12 occasional subantarctic species. A critical evaluation removed five species from the list, and one species has an unknown taxonomic status. The 42 Southern Ocean squids belong to three large taxonomic units, bathyteuthoids (n = 1 species), myopsids (n =1),andoegopsids (n = 40). A high level of endemism (21 species, 50%, all oegopsids) characterizes the Southern Ocean teuthofauna. Seventeen families of oegopsids are represented, with three dominating families, onychoteuthids (seven species, five endemics), ommastrephids (six species, three endemics), and cranchiids (five species, three endemics). Recent improvements in beak identification and taxonomy allowed making new correspondence between beak and species names, such as Galiteuthis suhmi (Hoyle 1886), Liguriella podophtalma Issel, 1908, and the recently described Taonius notalia Evans, in prep. Gonatus phoebetriae beaks were synonymized with those of Gonatopsis octopedatus Sasaki, 1920, thus increasing significantly the number of records and detailing the circumpolar distribution of this rarely caught Southern Ocean squid. The review extends considerably the number of species, including endemics, recorded from the Southern Ocean, but it also highlights that the corresponding species to two well-described beaks (Moroteuthopsis sp. -

Trace Element Analysis Reveals Bioaccumulation in the Squid Gonatus Fabricii from Polar Regions of the Atlantic Ocean A
Trace element analysis reveals bioaccumulation in the squid Gonatus fabricii from polar regions of the Atlantic Ocean A. Lischka, T. Lacoue-Labarthe, P. Bustamante, U. Piatkowski, H.J.T. Hoving To cite this version: A. Lischka, T. Lacoue-Labarthe, P. Bustamante, U. Piatkowski, H.J.T. Hoving. Trace element analysis reveals bioaccumulation in the squid Gonatus fabricii from polar regions of the Atlantic Ocean. Envi- ronmental Pollution, Elsevier, 2020, 256, pp.113389. 10.1016/j.envpol.2019.113389. hal-02412742 HAL Id: hal-02412742 https://hal.archives-ouvertes.fr/hal-02412742 Submitted on 15 Dec 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Trace element analysis reveals bioaccumulation in the squid Gonatus fabricii from polar regions of the Atlantic Ocean A. Lischka1*, T. Lacoue-Labarthe2, P. Bustamante2, U. Piatkowski3, H. J. T. Hoving3 1AUT School of Science New Zealand, Auckland University of Technology, Private Bag 92006, 1142, Auckland, New Zealand 2 Littoral Environnement et Sociétés (LIENSs), UMR 7266 CNRS-La Rochelle Université, 2 rue Olympe de Gouges, 17000 La Rochelle, France 3 GEOMAR, Helmholtz Centre for Ocean Research Kiel, Düsternbrooker Weg 20, 24105 Kiel, Germany *corresponding author: [email protected] 1 Abstract: The boreoatlantic gonate squid (Gonatus fabricii) represents important prey for top predators—such as marine mammals, seabirds and fish—and is also an efficient predator of crustaceans and fish. -

Translation 3204
4 of 6 I' rÉ:1°.r - - - Ï''.ec.n::::,- - — TRANSLATION 3204 and Van, else--- de ,-0,- SERIES NO(S) ^4p €'`°°'°^^`m`^' TRANSLATION 3204 5 of 6 serceaesoe^nee SERIES NO.(S) serv,- i°- I' ann., Canada ° '° TRANSLATION 3204 6 of 6 SERIES NO(S) • =,-""r I FISHERIES AND MARINE SERVICE ARCHIVE:3 Translation Series No. 3204 Multidisciplinary investigations of the continental slope in the Gulf of Alaska area by Z.A. Filatova (ed.) Original title: Kompleksnyye issledovaniya materikovogo sklona v raione Zaliva Alyaska From: Trudy Instituta okeanologii im. P.P. ShirshoV (Publications of the P.P. Shirshov Oceanpgraphy Institute), 91 : 1-260, 1973 Translated by the Translation Bureau(HGC) Multilingual Services Division Department of the Secretary of State of Canada Department of the Environment Fisheries and Marine Service Pacific Biological Station Nanaimo, B.C. 1974 ; 494 pages typescriPt "DEPARTMENT OF THE SECRETARY OF STATE SECRÉTARIAT D'ÉTAT TRANSLATION BUREAU BUREAU DES TRADUCTIONS MULTILINGUAL SERVICES DIVISION DES SERVICES DIVISION MULTILINGUES ceÔ 'TRANSLATED FROM - TRADUCTION DE INTO - EN Russian English Ain HOR - AUTEUR Z. A. Filatova (ed.) ri TL E IN ENGLISH - TITRE ANGLAIS Multidisciplinary investigations of the continental slope in the Gulf of Aâaska ares TI TLE IN FORE I GN LANGuAGE (TRANS LI TERA TE FOREIGN CHARACTERS) TITRE EN LANGUE ÉTRANGÈRE (TRANSCRIRE EN CARACTÈRES ROMAINS) Kompleksnyye issledovaniya materikovogo sklona v raione Zaliva Alyaska. REFERENCE IN FOREI GN LANGUAGE (NAME: OF BOOK OR PUBLICATION) IN FULL. TRANSLI TERATE FOREIGN CHARACTERS, RÉFÉRENCE EN LANGUE ÉTRANGÈRE (NOM DU LIVRE OU PUBLICATION), AU COMPLET, TRANSCRIRE EN CARACTÈRES ROMAINS. Trudy Instituta okeanologii im. P.P. -

An Illustrated Key to the Families of the Order
CLYDE F. E. ROP An Illustrated RICHARD E. YOl and GILBERT L. VC Key to the Families of the Order Teuthoidea Cephalopoda) SMITHSONIAN CONTRIBUTIONS TO ZOOLOGY • 1969 NUMBER 13 SMITHSONIAN CONTRIBUTIONS TO ZOOLOGY NUMBER 13 Clyde F. E. Roper, An Illustrated Key 5K?Z" to the Families of the Order Teuthoidea (Cephalopoda) SMITHSONIAN INSTITUTION PRESS CITY OF WASHINGTON 1969 SERIAL PUBLICATIONS OF THE SMITHSONIAN INSTITUTION The emphasis upon publications as a means of diffusing knowledge was expressed by the first Secretary of the Smithsonian Institution. In his formal plan for the Institution, Joseph Henry articulated a program that included the following statement: "It is proposed to publish a series of reports, giving an account of the new discoveries in science, and of the changes made from year to year in all branches of knowledge not strictly professional." This keynote of basic research has been adhered to over the years in the issuance of thousands of titles in serial publications under the Smithsonian imprint, commencing with Smithsonian Contributions to Knowledge in 1848 and continuing with the following active series: Smithsonian Annals of Flight Smithsonian Contributions to Anthropology Smithsonian Contributions to Astrophysics Smithsonian Contributions to Botany Smithsonian Contributions to the Earth Sciences Smithsonian Contributions to Paleobiology Smithsonian Contributions to Zoology Smithsonian Studies in History and Technology In these series, the Institution publishes original articles and monographs dealing with the research and collections of its several museums and offices and of professional colleagues at other institutions of learning. These papers report newly acquired facts, synoptic interpretations of data, or original theory in specialized fields. -

Arctic Cephalopod Distributions and Their Associated Predatorspor 146 209..227 Kathleen Gardiner & Terry A
Arctic cephalopod distributions and their associated predatorspor_146 209..227 Kathleen Gardiner & Terry A. Dick Biological Sciences, University of Manitoba, Winnipeg, Manitoba R3T 2N2, Canada Keywords Abstract Arctic Ocean; Canada; cephalopods; distributions; oceanography; predators. Cephalopods are key species of the eastern Arctic marine food web, both as prey and predator. Their presence in the diets of Arctic fish, birds and mammals Correspondence illustrates their trophic importance. There has been considerable research on Terry A. Dick, Biological Sciences, University cephalopods (primarily Gonatus fabricii) from the north Atlantic and the west of Manitoba, Winnipeg, Manitoba R3T 2N2, side of Greenland, where they are considered a potential fishery and are taken Canada. E-mail: [email protected] as a by-catch. By contrast, data on the biogeography of Arctic cephalopods are doi:10.1111/j.1751-8369.2010.00146.x still incomplete. This study integrates most known locations of Arctic cepha- lopods in an attempt to locate potential areas of interest for cephalopods, and the predators that feed on them. International and national databases, museum collections, government reports, published articles and personal communica- tions were used to develop distribution maps. Species common to the Canadian Arctic include: G. fabricii, Rossia moelleri, R. palpebrosa and Bathypolypus arcticus. Cirroteuthis muelleri is abundant in the waters off Alaska, Davis Strait and Baffin Bay. Although distribution data are still incomplete, groupings of cephalopods were found in some areas that may be correlated with oceanographic variables. Understanding species distributions and their interactions within the ecosys- tem is important to the study of a warming Arctic Ocean and the selection of marine protected areas. -

SARSIA EGG MASSES of the SQUID GONATUS FABRICII (CEPHALOPODA, GONATIDAE) CAUGHT with PELAGIC F TRAWL OFF NORTHERN NORWAY
SHORT COMMUNICATION SARSIA EGG MASSES OF THE SQUID GONATUS FABRICII (CEPHALOPODA, GONATIDAE) CAUGHT WITH PELAGIC f TRAWL OFF NORTHERN NORWAY ƒ H e r m a n B j0 r k e , K a r s t e n H a n s e n & R o l f C. S u n d t B j 0 r k e , H e r m a n , Ka r s t e n H a n s e n & R o l f C. S u n d t 1997 08 15. Egg masses o f the squidGonatus fabricii (Cephalopoda, Gonatidae) caught with pelagic trawl off northern Norway.Sarsia - 82:149- 152. Bergen. ISSN 0036-4827. A description of egg masses from Gonatus fabricii (L ichtenstein ) caught with pelagic trawl in the Norwegian Sea is given. The eggs were kept together in a single layer between two mucous mem branes, and the pieces collected appeared to be fragments of more extensive structures tom apart by wear from the sampling gear. No embryos were observed in the eggs, and none of the eggs showed any staining for five enzyme systems analyzed by isoelectric focusing. Either the eggs were caught shortly after spawning and fertilization, or the lack of embryonic tissue reflects the fact that most of the eggs were caught in water colder than 0 °C. The developmental rate at this temperature is ex pected to be very slow. Herman Bjorke, Karsten Hansen & Rolf C. Sundt, Institute of Marine Research, P.O. Box 1870 Nordnes, N-5024 Bergen, Norway. K e y w o r d s : Cephalopoda;Gonatus fabricii', Norwegian Sea; egg masses; pelagic trawl. -

1995 K12.Pdf
• This paper not to be cited without prior reference to the author International Council for CM 1995/K:12 Ref. N the exploration of the Sea Shellfish Committee NORWEGIAN INVESTIGATIONS OM GONATUS FABRICII (LICHTENSTEIN) by Herman Bjorke Institute of Marine Research P. O. Box 1870 Nordnes N-5024 Bergen, Norway ABSTRACT • By far the majority of the investigated GonatUs fabricii were caught as bycatch by pelagic trawls fishing .in the upper 60 m. Sampling took place in April/May,June/July and August/September in the period 1978 1991. The distribution of gonatus along the Norwegian coast south of 71oN is clearly connected with Atlantic water with salinities above 35°;00. The highest concentrations are found in the area between Jan Mayen and Spitsbergen, i.e. in the Polar front area. The biomass oE younggonatus in the Norwegian Sea constituted at least 1.5 mill tonnes in July 1994. Young gonatus begin to occur in thesurface layers in May, and they seem to descend from the upper 60 m at a length of50-60 mm. They rnight then live near the bottom or pelagically at depths of 200-600 m.. Age and growth based on counts of primary growth rings should be treated with caution. At least in the eastem part of the Norwegian Sea prey iteins for gonatus are, in decreasing order of impoerance, amphipods, mainly Parathemisto spp., copepods, chaetognaths and euphausiids. Larger gonatus consume fry of Sebastes sp., Maurolicus mülleri, and small gonatus. • Based on catch statistics are four spawning areas for gonatus suggested: West of Spitsbergen, off Vesterälen, off More and between Iceland and Jan Mayen. -
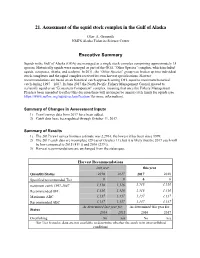
Assessment of the Squid Stock Complex in the Gulf of Alaska
21. Assessment of the squid stock complex in the Gulf of Alaska Olav A. Ormseth NMFS Alaska Fisheries Science Center Executive Summary Squids in the Gulf of Alaska (GOA) are managed as a single stock complex comprising approximately 15 species. Historically squids were managed as part of the GOA “Other Species” complex, which included squids, octopuses, sharks, and sculpins. In 2011, the “Other Species” group was broken up into individual stock complexes and the squid complex received its own harvest specifications. Harvest recommendations are based on an historical catch approach setting OFL equal to maximum historical catch during 1997 – 2007. In June 2017 the North Pacific Fishery Management Council moved to reclassify squid as an “Ecosystem Component” complex, meaning that once the Fishery Management Plan has been amended to reflect this decision there will no longer be annual catch limits for squids (see https://www.npfmc.org/squid-reclassification/ for more information). Summary of Changes in Assessment Inputs 1) Trawl survey data from 2017 have been added. 2) Catch data have been updated through October 11, 2017. Summary of Results 1) The 2017 trawl survey biomass estimate was 2,296 t, the lowest it has been since 1999. 2) The 2017 catch data are incomplete (29 t as of October 11), but it is likely that the 2017 catch will be low compared to 2015 (411 t) and 2016 (239 t). 3) Harvest recommendations are unchanged from the status quo. Harvest Recommendations last year this year Quantity/Status 2016 2017 2017 2018 Specified/recommended -

Giant Protistan Parasites on the Gills of Cephalopods (Mollusca)
DISEASES OF AQUATIC ORGANISMS Vol. 3: 119-125. 1987 Published December 14 Dis. aquat. Org. Giant protistan parasites on the gills of cephalopods (Mollusca) Norman ~c~ean',F. G. ~ochberg~,George L. shinn3 ' Biology Department, San Diego State University, San Diego, California 92182-0057, USA Department of Invertebrate Zoology, Santa Barbara Museum of Natural History, 2559 Puesta Del Sol Road, Santa Barbara, California 93105. USA Division of Science, Northeast Missouri State University, Kirksville. Missouri 63501, USA ABSTRACT: Large Protista of unknown taxonomic affinities are described from 3 species of coleoid squids, and are reported from many other species of cephalopods. The white to yellow-orange, ovoid cyst-like parasites are partially embedded within small pockets on the surface of the gills, often in large numbers. Except for a holdfast region on one side of the large end, the surface of the parasite is elaborated into low triangular plates separated by grooves. The parasites are uninucleate; their cytoplasm bears lipid droplets and presumed paraglycogen granules. Trichocysts, present in a layer beneath the cytoplasmic surface, were found by transmission electron microscopy to be of the dino- flagellate type. Further studies are needed to clarify the taxonomic position of these protists. INTRODUCTION epoxy resin (see below). One specimen each of the coleoid squids Abralia trigonura and Histioteuthis dof- Cephalopods harbor a diversity of metazoan and leini were trawled near Oahu, Hawaii, in March, 1980. protozoan parasites (Hochberg 1983). In this study we Gill parasites from the former were fixed in formalin; used light and electron microscopy to characterize a those from the latter were fixed in osmium tetroxide. -

2011 GOA Squids
21. Gulf of Alaska squids Olav A. Ormseth NMFS Alaska Fisheries Science Center Executive Summary In 2010, the North Pacific Fishery Management Council passed amendment 87 to the GOA Fishery Management Plan, which separated the Other Species complex into its constituent species groups. Thus, GOA squids are now managed as an independent complex with its own harvest specifications. Because the GOA bottom trawl survey is the chief source of data for this assessment and is a biennial survey, full assessments are performed only in odd years. Summary of Changes Changes in the input data: 1. Total catch and retention data for GOA squids has been updated with complete 2010 and partial 2011 data. 2. Biomass estimates from the 2011 GOA bottom trawl survey have been added. 3. An appendix containing data regarding non-commercial catches of squid has been added to the report. Summary of Results Because reliable estimates of squid biomass and natural mortality rate do not exist, we recommend using a modified Tier 6 approach setting OFL equal to maximum historical catch and ABC equal to 0.75 * OFL using the years 1997 - 2007 as a baseline . last year this year Quantity/Status 2011 2012 2012 2013 M (natural mortality) n/a n/a n/a n/a Specified/recommended Tier 6 6 6 6 Biomass n/a n/a n/a n/a average historical catch 1997-2007 272 272 272 272 maximum historical catch 1997-2007 1,530 1,530 1,530 1,530 Recommended OFL (max. hist. catch; t) 1,530 1,530 1,530 1,530 Recommended ABC (0.75*OFL; t) 1,148 1,148 1,148 1,148 As determined last As determined this Status year for: year for: 2009 2010 2010 2011 Overfishing No n/a No n/a Overfished n/a n/a n/a n/a Approaching overfished n/a n/a n/a n/a (for Tier 6 stocks, data are not available to determine whether the stock is in an overfished condition) Introduction Description, scientific names, and general distribution Squids (order Teuthoidea) are cephalopod molluscs which are related to octopus. -

SYSTEMATICS of the CEPHALOPOD FAMILY GONATIDAE from the SOUTHEASTERN BERING SEA RECOMMENDED: APPROVED: F — > Program Head V
Systematics of the Cephalopod family Gonatidae from the southeastern Bering Sea Item Type Thesis Authors Bublitz, Christopher G. Download date 06/10/2021 03:42:26 Link to Item http://hdl.handle.net/11122/5209 SYSTEMATICS OF THE CEPHALOPOD FAMILY GONATIDAE FROM THE SOUTHEASTERN BERING SEA RECOMMENDED: f — > Programin i Head. APPROVED: Vice Chancellor ror Research and Advanced Study Date (J SYSTEMATICS OF THE CEPHALOPOD FAMILY GONATIDAE FROM THE SOUTHEASTERN BERING SEA A THESIS Presented to the Faculty of the University of Alaska in Partial Fulfillment of the Requirements for the Degree of MASTER OF SCIENCE By Qu Christopher G. Bublitz 4- o Fairbanks, Alaska '3 < o May 1981 H ABSTRACT The systematic relationships within the cephalopod family Gonatidae were examined utilizing specimens collected from the southeastern Bering Sea. Ten species; Gonatus onyx, Gonatus madokai, Gonatus tinvo3 Gonatus bewyi, Gonatus pyvos3 Gonatus sp., Gonatus type A, BevvyteuthLs magistev3 Berryteuthis anonychus3 and Gonatopsis bovealis; were identified. Included in these are the identification of a probable new species, Gonatus sp., the verification of a questionable species, Gonatus tinro, and the classification of previously described but unclassified developmental stages. Morphometric characters were used for a retrogression analysis of each species' development. The developmental stages for those species found in the study area are described and illustrated in detail. The taxonomic and morphometric characters of the species are compared. Two closely allied species, Gonatus bevvyi and Gonatus sp. are compared and contrasted. A brief an§J.ysis of the growth and development of the genus Gonatus is also given. ACKNOWLEDGEMENT S I gratefully acknowledge the assistance and encouragement given me by a large number of people, both in the United States and Japan. -

Appendix E Marine Mammal Density Report
Appendix E Marine Mammal Density Report GULF OF ALASKA NAVY TRAINING ACTIVITIES EIS/OEIS FINAL (MARCH 2011) TABLE OF CONTENTS E MARINE MAMMAL DENSITY AND DEPTH DISTRIBUTION .................................... E-1 E.1 BACKGROUND AND OVERVIEW .............................................................................................. E-1 E.1.1 DENSITY ................................................................................................................................. E-1 E.1.2 DEPTH DISTRIBUTION ......................................................................................................... E-6 E.1.3 DENSITY AND DEPTH DISTRIBUTION COMBINED ....................................................... E-6 E.2 MYSTICETES ............................................................................................................................ E-7 E.2.1 BLUE WHALE, BALAENOPTERA MUSCULUS ................................................................... E-7 E.2.2 FIN WHALE, BALAENOPTERA PHYSALUS ......................................................................... E-8 E.2.3 SEI WHALE, BALAENOPTERA BOREALIS........................................................................... E-8 E.2.4 MINKE WHALE, BALAENOPTERA ACUTOROSTRATA ..................................................... E-8 E.2.5 HUMPBACK WHALE, MEGAPTERA NOVAEANGLIAE ..................................................... E-9 E.2.6 NORTH PACIFIC RIGHT WHALE, EUBALAENA JAPONICA ............................................ E-9 E.2.7 GRAY WHALE, ESCHRICHTIUS