Glaciers, Sea Ice, and Ice Formation (Dynamic Earth)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Numerical Modelling of Snow and Ice Thicknesses in Lake Vanajavesi, Finland
View metadata, citation and similar papers at core.ac.uk SERIES A brought to you by CORE DYNAMIC METEOROLOGY provided by Helsingin yliopiston digitaalinen arkisto AND OCEANOGRAPHY PUBLISHED BY THE INTERNATIONAL METEOROLOGICAL INSTITUTE IN STOCKHOLM Numerical modelling of snow and ice thicknesses in Lake Vanajavesi, Finland By YU YANG1,2*, MATTI LEPPA¨ RANTA2 ,BINCHENG3,1 and ZHIJUN LI1, 1State Key Laboratory of Coastal and Offshore Engineering, Dalian University of Technology, Dalian 116024, China; 2Department of Physics, University of Helsinki, PO Box 48, FI-00014 Helsinki, Finland; 3Finnish Meteorological Institute, PO Box 503, FI-00101, Helsinki, Finland (Manuscript received 27 March 2011; in final form 7 January 2012) ABSTRACT Snow and ice thermodynamics was simulated applying a one-dimensional model for an individual ice season 2008Á2009 and for the climatological normal period 1971Á2000. Meteorological data were used as the model input. The novel model features were advanced treatment of superimposed ice and turbulent heat fluxes, coupling of snow and ice layers and snow modelled from precipitation. The simulated snow, snowÁice and ice thickness showed good agreement with observations for 2008Á2009. Modelled ice climatology was also reasonable, with 0.5 cm d1 growth in DecemberÁMarch and 2 cm d1 melting in April. Tuned heat flux from waterto ice was 0.5 W m 2. The diurnal weather cycle gave significant impact on ice thickness in spring. Ice climatology was highly sensitive to snow conditions. Surface temperature showed strong dependency on thickness of thin ice (B0.5 m), supporting the feasibility of thermal remote sensing and showing the importance of lake ice in numerical weather prediction. -

Local Topography Increasingly Influences the Mass Balance of A
Local topography increasingly influences the mass balance of a retreating cirque glacier Caitlyn Florentine1, 2, Joel Harper2, Daniel Fagre1, Johnnie Moore2, Erich Peitzsch1 1U.S. Geological Survey, Northern Rocky Mountain Science Center, West Glacier, Montana, 59936, USA 5 2Department of Geosciences, University of Montana, Missoula, Montana, 59801, USA Correspondence to: Caitlyn Florentine ([email protected]) Abstract. Local topographically driven processes, such as wind drifting, avalanching, and shading, are known to alter the relationship between the mass balance of small cirque glaciers and regional climate. Yet partitioning such local effects from regional climate influence has proven difficult, creating uncertainty in the climate representativeness of some glaciers. We 10 address this problem for Sperry Glacier in Glacier National Park, USA using field-measured surface mass balance, geodetic constraints on mass balance, and regional climate data recorded at a network of meteorological and snow stations. Geodetically derived mass changes between 1950-1960, 1960-2005, and 2005-2014 document average mass change rates during each period at -0.22±0.12 m w.e. yr-1, -0.18±0.05 m w.e. yr-1, and -0.10±0.03 m w.e. yr-1. A correlation of field- measured mass balance and regional climate variables closely (i.e. within 0.08 m w.e. yr-1) predicts the geodetically 15 measured mass loss from 2005-2014. However, this correlation overestimates glacier mass balance for 1950-1960 by +1.18±0.92 m w.e. yr-1. Our analysis suggests that local effects, not represented in regional climate variables, have become a more dominant driver of the net mass balance as the glacier lost 0.50 km2 and retreated further into its cirque. -

Characteristics of the Bergschrund of an Avalanche-Cone Glacier in the Canadian Rocky Mountains
JOlIl"lla/ o/G/aci%gl'. VoL 29. No. 10 1. 1983 CHARACTERISTICS OF THE BERGSCHRUND OF AN AVALANCHE-CONE GLACIER IN THE CANADIAN ROCKY MOUNTAINS By G ERALD OSBORN (Department of Geology and Geophysics, Uni versity o f Calgary, Calgary, Alberta T2N I N4, Canada) ABSTRACT. Fi eld study of th e bergschrund of a small avalanche-cone glacier at the base of Mt Chephren, in Banff Nati onal Park , has been ca rried out as part of a general ex pl oratory study of glacier-head crevasses in th e Canadi an Roc ki es. The bergsc hrun d consists of a wide. shall ow. partl y bedrock-fl oored gap, und erneath whi ch ex tends a nearl y vertical Ralldklu!I, and a small , offset, subsidi ary crevasse (or crevasses). The fo ll owin g observations rega rdin g the behavior of th e bergsc hruncl and ice adjacent to it are of parti cul ar interest: ( I) topograph y of the subglaeial bedrock is a control on the location of the main bergschrund and subsidi a ry crevasses. (2) th e main bergschrund and subsid ia ry crevasse(s) are conn ected by subglacial gaps betwee n bedrock and ice; th e gaps are part of th e "bergschrund system" , (3) snow/ ice immedi ately down-glacier of the bergschrund system moves nea rl y verticall y dow nwa rd in response to rotational fl ow of the glacier. a ll owin g the bergschrund components to keep the same location and size fro m year to year, (4) an inde pend ent accumul ati on, fl ow. -

Geomorphic Character, Age and Distribution of Rock Glaciers in the Olympic Mountains, Washington
Portland State University PDXScholar Dissertations and Theses Dissertations and Theses 1987 Geomorphic character, age and distribution of rock glaciers in the Olympic Mountains, Washington Steven Paul Welter Portland State University Follow this and additional works at: https://pdxscholar.library.pdx.edu/open_access_etds Part of the Geology Commons, and the Geomorphology Commons Let us know how access to this document benefits ou.y Recommended Citation Welter, Steven Paul, "Geomorphic character, age and distribution of rock glaciers in the Olympic Mountains, Washington" (1987). Dissertations and Theses. Paper 3558. https://doi.org/10.15760/etd.5440 This Thesis is brought to you for free and open access. It has been accepted for inclusion in Dissertations and Theses by an authorized administrator of PDXScholar. For more information, please contact [email protected]. AN ABSTRACT OF THE THESIS OF Steven Paul Welter for the Master of Science in Geography presented August 7, 1987. Title: The Geomorphic Character, Age, and Distribution of Rock Glaciers in the Olympic Mountains, Washington APPROVED BY MEMBERS OF THE THESIS COMMITTEE: Rock glaciers are tongue-shaped or lobate masses of rock debris which occur below cliffs and talus in many alpine regions. They are best developed in continental alpine climates where it is cold enough to preserve a core or matrix of ice within the rock mass but insufficiently snowy to produce true glaciers. Previous reports have identified and briefly described several rock glaciers in the Olympic Mountains, Washington {Long 1975a, pp. 39-41; Nebert 1984), but no detailed integrative study has been made regarding the geomorphic character, age, 2 and distribution of these features. -

Hnitflrcitilc
HNiTflRCiTilC A NEWS BULLETIN published quarterly by the NEW ZEALAND ANTARCTIC SOCIETY (INC) ,m — i * Halley, the British Antarctic Survey's station on the Brunt Ice Shelf, Coats Land,, was rebuilt last season for the third time since 1956-57. This picture taken in March shows one of the four wooden tubes, each of which houses a two-storey building, under construction in a pre-shaped and compacted snow hollow. BAS Copyngh! Registered at Post Office Headquarters, Vol. 10, No. 2 Wellington, New Zealand, as a magazine. SOUTH GEORGIA -.. SOUTH SANDWICH Is «C*2K SOUTH ORKNEY Is x \ 6SignyluK //o Orcadas arg SOUTH AMERICA / /\ ^ Borga T"^00Molodezhnaya \^' 4 south , * /weooEii \ ft SA ' r-\ *r\USSR --A if SHETLAND ,J£ / / ^^Jf ORONMIIDROWNING MAUD LAND' E N D E R B Y \ ] > * \ /' _ "iV**VlX" JN- S VDruzhnaya/General /SfA/ S f Auk/COATS ' " y C O A TBelirano SLd L d l arg L A N D p r \ ' — V&^y D««hjiaya/cenera.1 Beld ANTARCTIC •^W^fCN, uSS- fi?^^ /K\ Mawson \ MAC ROBERTSON LAN0\ \ *usi \ /PENINSULA' ^V^/^CRp^e J ^Vf (set mjp Mow) C^j V^^W^gSobralARG - Davis aust L Siple USA Amundsen-Scon OUEEN MARY LAND flMimy ELLSWORTH , U S A / ^ U S S R ') LAND °Vos1okussR/ r». / f c i i \ \ MARIE BYRO fee Shelf V\ . IAND WILKES LAND Scon ROSS|N2i? SEA jp>r/VICTORIAIj^V .TERRE ,; ' v / I ALAND n n \ \^S/ »ADEUL. n f i i f / / GEORGE V Ld .m^t Dumom d'Urville iranu Leningradskayra V' USSR,.'' \ -------"'•BAlLENYIs^ ANTARCTIC PENINSULA 1 Teniente Matienzo arc 2 Esperanza arg 3 Almirante Brown arg 4 Petrel arg 5 Decepcion arg 6 Vicecomodoro Marambio arg ' ANTARCTICA 7 Ariuro Prat chile 500 1000 Miles 8 Bernardo O'Higgms chile 9 Presidente Frei chile - • 1000 Kilomnre 10 Stonington I. -

2Growth, Structure and Properties of Sea
Growth, Structure and Properties 2 of Sea Ice Chris Petrich and Hajo Eicken 2.1 Introduction The substantial reduction in summer Arctic sea ice extent observed in 2007 and 2008 and its potential ecological and geopolitical impacts generated a lot of attention by the media and the general public. The remote-sensing data documenting such recent changes in ice coverage are collected at coarse spatial scales (Chapter 6) and typically cannot resolve details fi ner than about 10 km in lateral extent. However, many of the processes that make sea ice such an important aspect of the polar oceans occur at much smaller scales, ranging from the submillimetre to the metre scale. An understanding of how large-scale behaviour of sea ice monitored by satellite relates to and depends on the processes driving ice growth and decay requires an understanding of the evolution of ice structure and properties at these fi ner scales, and is the subject of this chapter. As demonstrated by many chapters in this book, the macroscopic properties of sea ice are often of most interest in studies of the interaction between sea ice and its environment. They are defi ned as the continuum properties averaged over a specifi c volume (Representative Elementary Volume) or mass of sea ice. The macroscopic properties are determined by the microscopic structure of the ice, i.e. the distribution, size and morphology of ice crystals and inclusions. The challenge is to see both the forest, i.e. the role of sea ice in the environment, and the trees, i.e. the way in which the constituents of sea ice control key properties and processes. -

Chronology, Stable Isotopes, and Glaciochemistry of Perennial Ice in Strickler Cavern, Idaho, USA
Investigation of perennial ice in Strickler Cavern, Idaho, USA Chronology, stable isotopes, and glaciochemistry of perennial ice in Strickler Cavern, Idaho, USA Jeffrey S. Munroe†, Samuel S. O’Keefe, and Andrew L. Gorin Geology Department, Middlebury College, Middlebury, Vermont 05753, USA ABSTRACT INTRODUCTION in successive layers of cave ice can provide a record of past changes in atmospheric circula- Cave ice is an understudied component The past several decades have witnessed a tion (Kern et al., 2011a). Alternating intervals of of the cryosphere that offers potentially sig- massive increase in research attention focused ice accumulation and ablation provide evidence nificant paleoclimate information for mid- on the cryosphere. Work that began in Antarc- of fluctuations in winter snowfall and summer latitude locations. This study investigated tica during the first International Geophysi- temperature over time (e.g., Luetscher et al., a recently discovered cave ice deposit in cal Year in the late 1950s (e.g., Summerhayes, 2005; Stoffel et al., 2009), and changes in cave Strickler Cavern, located in the Lost River 2008), increasingly collaborative efforts to ex- ice mass balances observed through long-term Range of Idaho, United States. Field and tract long ice cores from Antarctica (e.g., Jouzel monitoring have been linked to weather patterns laboratory analyses were combined to de- et al., 2007; Petit et al., 1999) and Greenland (Schöner et al., 2011; Colucci et al., 2016). Pol- termine the origin of the ice, to limit its age, (e.g., Grootes et al., 1993), satellite-based moni- len and other botanical evidence incorporated in to measure and interpret the stable isotope toring of glaciers (e.g., Wahr et al., 2000) and the ice can provide information about changes compositions (O and H) of the ice, and to sea-ice extent (e.g., Serreze et al., 2007), field in surface environments (Feurdean et al., 2011). -

Mountain-Derived Versus Shelf-Based Glaciations on the Western Taymyr Peninsula, Siberia Christian Hjort1 & Svend Funder2
Mountain-derived versus shelf-based glaciations on the western Taymyr Peninsula, Siberia Christian Hjort1 & Svend Funder2 1 Quaternary Sciences, Lund University, GeoCenter II, Sölvegatan 12, SE-223 62 Lund, Sweden 2 Natural History Museum, University of Copenhagen, Øster Voldgade 5-7, DK-1350 Copenhagen K, Denmark Keywords Abstract Siberian geology; glacial inception; glacial history. The early Russian researchers working in central Siberia seem to have preferred scenarios in which glaciations, in accordance with the classical gla- Correspondence ciological concept, originated in the mountains. However, during the last 30 C. Hjort, Quaternary Sciences, Lund years or so the interest in the glacial history of the region has concentrated on University, GeoCenter II, Sölvegatan 12, ice sheets spreading from the Kara Sea shelf. There, they could have originated SE-223 62 Lund, Sweden. E-mail: from ice caps formed on areas that, for eustatic reasons, became dry land [email protected] during global glacial maximum periods, or from grounded ice shelves. Such ice doi:10.1111/j.1751-8369.2008.00068.x sheets have been shown to repeatedly inundate much of the Taymyr Peninsula from the north-west. However, work on westernmost Taymyr has now also documented glaciations coming from inland. On at least two occasions, with the latest one dated to the Saale glaciation (marine isotope stage 6 [MIS 6]), warm-based, bedrock-sculpturing glaciers originating in the Byrranga Moun- tains, and in the hills west of the range, expanded westwards, and at least once did such glaciers, after moving 50–60 km or more over the present land areas, cross today’s Kara Sea coastline. -

The Immediate Paraglacial Response of Deglaciating Cirque Walls
Current glacier recession causes significant rockfall increase: The immediate paraglacial response of deglaciating cirque walls Ingo Hartmeyer1, Robert Delleske1, Markus Keuschnig1, Michael Krautblatter2, Andreas Lang3, Lothar Schrott4, Jan-Christoph Otto3 5 1GEORESEARCH Research Institute, Wals, 5071, Austria 2Chair of Landslide Research, Technical University of Munich, Munich, 80333, Germany 3Department of Geography and Geology, University of Salzburg, Salzburg, 5020, Austria 4Department of Geography, University of Bonn, Bonn, 53115, Germany Correspondence to: Ingo Hartmeyer ([email protected]) 10 Abstract. In the European Alps almost half the glacier volume disappeared over the past 150 years. The loss is reflected in glacier retreat and ice surface lowering even at high altitude. In steep glacial cirques surface lowering exposes rock to atmospheric conditions probably for the very first time in several millennia. Instability of rockwalls has long been identified as one of the direct consequences of deglaciation, but so far cirque-wide quantification of rockfall at high-resolution is missing. Based on terrestrial LiDAR a rockfall inventory for the permafrost-affected rockwalls of two rapidly deglaciating cirques in 15 the Central Alps of Austria (Kitzsteinhorn) is established. Over six-years (2011-2017) 78 rockwall scans were acquired to generate data of high spatial and temporal resolution. 632 rockfalls were registered ranging from 0.003 to 879.4 m³, mainly originating from pre-existing structural rock weaknesses. 60 % of the rockfall volume detached from less than ten vertical meters above the glacier surface, indicating enhanced rockfall activity over tens of years following deglaciation. Debuttressing seems to play a minor effect only. Rather, preconditioning is assumed to start inside the Randkluft (void between cirque wall 20 and glacier) where measured sustained freezing and ample supply of liquid water likely cause enhanced physical weathering and high quarrying stresses. -

The Periglacial Climate and Environment in Northern Eurasia
ARTICLE IN PRESS Quaternary Science Reviews 23 (2004) 1333–1357 The periglacial climate andenvironment in northern Eurasia during the Last Glaciation Hans W. Hubbertena,*, Andrei Andreeva, Valery I. Astakhovb, Igor Demidovc, Julian A. Dowdeswelld, Mona Henriksene, Christian Hjortf, Michael Houmark-Nielseng, Martin Jakobssonh, Svetlana Kuzminai, Eiliv Larsenj, Juha Pekka Lunkkak, AstridLys a(j, Jan Mangerude, Per Moller. f, Matti Saarnistol, Lutz Schirrmeistera, Andrei V. Sherm, Christine Siegerta, Martin J. Siegertn, John Inge Svendseno a Alfred Wegener Institute for Polar and Marine Research (AWI), Telegrafenberg A43, Potsdam D-14473, Germany b Geological Faculty, St. Petersburg University, Universitetskaya 7/9, St. Petersburg 199034, Russian Federation c Institute of Geology, Karelian Branch of Russian Academy of Sciences, Pushkinskaya 11, Petrozavodsk 125610, Russian Federation d Scott Polar Research Institute and Department of Geography, University of Cambridge, Cambridge CBZ IER, UK e Department of Earth Science, University of Bergen, Allegt.! 41, Bergen N-5007, Norway f Quaternary Science, Department of Geology, Lund University, Geocenter II, Solvegatan. 12, Lund Sweden g Geological Institute, University of Copenhagen, Øster Voldgade 10, Copenhagen DK-1350, Denmark h Center for Coastal and Ocean Mapping, Chase Ocean Engineering Lab, University of New Hampshire, Durham, NH 03824, USA i Paleontological Institute, RAS, Profsoyuznaya ul., 123, Moscow 117868, Russia j Geological Survey of Norway, PO Box 3006 Lade, Trondheim N-7002, Norway -

1 Compiled by Mike Wing New Zealand Antarctic Society (Inc
ANTARCTIC 1 Compiled by Mike Wing US bulldozer, 1: 202, 340, 12: 54, New Zealand Antarctic Society (Inc) ACECRC, see Antarctic Climate & Ecosystems Cooperation Research Centre Volume 1-26: June 2009 Acevedo, Capitan. A.O. 4: 36, Ackerman, Piers, 21: 16, Vessel names are shown viz: “Aconcagua” Ackroyd, Lieut. F: 1: 307, All book reviews are shown under ‘Book Reviews’ Ackroyd-Kelly, J. W., 10: 279, All Universities are shown under ‘Universities’ “Aconcagua”, 1: 261 Aircraft types appear under Aircraft. Acta Palaeontolegica Polonica, 25: 64, Obituaries & Tributes are shown under 'Obituaries', ACZP, see Antarctic Convergence Zone Project see also individual names. Adam, Dieter, 13: 6, 287, Adam, Dr James, 1: 227, 241, 280, Vol 20 page numbers 27-36 are shared by both Adams, Chris, 11: 198, 274, 12: 331, 396, double issues 1&2 and 3&4. Those in double issue Adams, Dieter, 12: 294, 3&4 are marked accordingly. Adams, Ian, 1: 71, 99, 167, 229, 263, 330, 2: 23, Adams, J.B., 26: 22, Adams, Lt. R.D., 2: 127, 159, 208, Adams, Sir Jameson Obituary, 3: 76, A Adams Cape, 1: 248, Adams Glacier, 2: 425, Adams Island, 4: 201, 302, “101 In Sung”, f/v, 21: 36, Adamson, R.G. 3: 474-45, 4: 6, 62, 116, 166, 224, ‘A’ Hut restorations, 12: 175, 220, 25: 16, 277, Aaron, Edwin, 11: 55, Adare, Cape - see Hallett Station Abbiss, Jane, 20: 8, Addison, Vicki, 24: 33, Aboa Station, (Finland) 12: 227, 13: 114, Adelaide Island (Base T), see Bases F.I.D.S. Abbott, Dr N.D. -
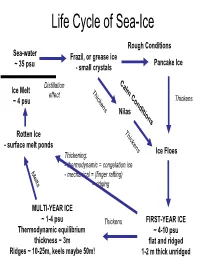
Life Cycle of Sea-Ice
Life Cycle of Sea-Ice Rough Conditions Sea-water Frazil, or grease ice Pancake Ice ~ 35 psu - small crystals C Distillation a Ice Melt T lm effect hi C ~ 4 psu ck o Thickens ens nd Nilas itio ns T hi Rotten Ice c - surface melt ponds kens Ice Floes Thickening: - thermodynamic = congelation ice M e - mechanical = (finger rafting) l ts = ridging MULTI-YEAR ICE ~ 1-4 psu Thickens FIRST-YEAR ICE Thermodynamic equilibrium ~ 4-10 psu thickness ~ 3m flat and ridged Ridges ~ 10-25m, keels maybe 50m! 1-2 m thick unridged Internal Structure of Sea Ice Brine Channels within the ice (~width of human hair) Brine rejected from ice (4-10psu), away from surface, but concentrates in brine channels long crystals as congelation ice (small volume but VERY HIGH SALINITIES) (frozen on from below) -6 deg C -10 deg C -21 deg C 100psu 145psu 216psu Pictures from AWI Brine Volume and Salinity From Thomas and Dieckmann 2002, Science .... adapted from papers by Hajo Eichen Impacts of Sea-ice on the Ocean ICE FORMATION and PRESENCE Wind - brine rejection - Ocean-Atmos momentum barrier - Ocean-Atmos heat barrier - ice edge processes (e.g. upwelling) - keel stirring (i.e. mixing, but < wind) Ocean 10psu MELTING ICE Fresh 35psu - stratification (fresher water) (cf. distillation as ice moves from formation region) Saltier - transport of sediment, etc S increases START FREEZE MELT Impacts of Sea-ice on the Atmosphere ICE PRESENCE - albedo change - Ocean-Atmos momentum barrier - Ocean-Atmos heat barrier Water Sky Sea Smoke Heat balance S=Shortwave radiation from sun (reflects off clouds and surface) albedo= how much radiation reflects from surface albedo of ice ~ 0.8 albedo of water ~ 0.04 (if sun overhead) L=Longwave radiation (from surface and clouds) F=Heat flux from Ocean M=Melt (snow and ice) From N.