Frazil Ice Formation in the Polar Oceans
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Numerical Modelling of Snow and Ice Thicknesses in Lake Vanajavesi, Finland
View metadata, citation and similar papers at core.ac.uk SERIES A brought to you by CORE DYNAMIC METEOROLOGY provided by Helsingin yliopiston digitaalinen arkisto AND OCEANOGRAPHY PUBLISHED BY THE INTERNATIONAL METEOROLOGICAL INSTITUTE IN STOCKHOLM Numerical modelling of snow and ice thicknesses in Lake Vanajavesi, Finland By YU YANG1,2*, MATTI LEPPA¨ RANTA2 ,BINCHENG3,1 and ZHIJUN LI1, 1State Key Laboratory of Coastal and Offshore Engineering, Dalian University of Technology, Dalian 116024, China; 2Department of Physics, University of Helsinki, PO Box 48, FI-00014 Helsinki, Finland; 3Finnish Meteorological Institute, PO Box 503, FI-00101, Helsinki, Finland (Manuscript received 27 March 2011; in final form 7 January 2012) ABSTRACT Snow and ice thermodynamics was simulated applying a one-dimensional model for an individual ice season 2008Á2009 and for the climatological normal period 1971Á2000. Meteorological data were used as the model input. The novel model features were advanced treatment of superimposed ice and turbulent heat fluxes, coupling of snow and ice layers and snow modelled from precipitation. The simulated snow, snowÁice and ice thickness showed good agreement with observations for 2008Á2009. Modelled ice climatology was also reasonable, with 0.5 cm d1 growth in DecemberÁMarch and 2 cm d1 melting in April. Tuned heat flux from waterto ice was 0.5 W m 2. The diurnal weather cycle gave significant impact on ice thickness in spring. Ice climatology was highly sensitive to snow conditions. Surface temperature showed strong dependency on thickness of thin ice (B0.5 m), supporting the feasibility of thermal remote sensing and showing the importance of lake ice in numerical weather prediction. -

River Ice Management in North America
RIVER ICE MANAGEMENT IN NORTH AMERICA REPORT 2015:202 HYDRO POWER River ice management in North America MARCEL PAUL RAYMOND ENERGIE SYLVAIN ROBERT ISBN 978-91-7673-202-1 | © 2015 ENERGIFORSK Energiforsk AB | Phone: 08-677 25 30 | E-mail: [email protected] | www.energiforsk.se RIVER ICE MANAGEMENT IN NORTH AMERICA Foreword This report describes the most used ice control practices applied to hydroelectric generation in North America, with a special emphasis on practical considerations. The subjects covered include the control of ice cover formation and decay, ice jamming, frazil ice at the water intakes, and their impact on the optimization of power generation and on the riparians. This report was prepared by Marcel Paul Raymond Energie for the benefit of HUVA - Energiforsk’s working group for hydrological development. HUVA incorporates R&D- projects, surveys, education, seminars and standardization. The following are delegates in the HUVA-group: Peter Calla, Vattenregleringsföretagen (ordf.) Björn Norell, Vattenregleringsföretagen Stefan Busse, E.ON Vattenkraft Johan E. Andersson, Fortum Emma Wikner, Statkraft Knut Sand, Statkraft Susanne Nyström, Vattenfall Mikael Sundby, Vattenfall Lars Pettersson, Skellefteälvens vattenregleringsföretag Cristian Andersson, Energiforsk E.ON Vattenkraft Sverige AB, Fortum Generation AB, Holmen Energi AB, Jämtkraft AB, Karlstads Energi AB, Skellefteå Kraft AB, Sollefteåforsens AB, Statkraft Sverige AB, Umeå Energi AB and Vattenfall Vattenkraft AB partivipates in HUVA. Stockholm, November 2015 Cristian -

Sea Ice Growth and Decay a Remote Sensing Perspective Sea Ice Growth & Decay
Sea Ice Growth And Decay A Remote Sensing Perspective Sea Ice Growth & Decay Add snow here Loss of Sea Ice in the Arctic Donald K. Perovich and Jacqueline A. Richter-Menge Annual Review of Marine Science 2009 1:1, 417-441 Sea Ice Remote Sensing Method Physical Property Sensor Type Geophysical Variable Passive Microwave Surface Emissivity Radiometer Sea Ice Concentration ~ 1 – 100 GHz Sea Ice Classification Sea Ice Motion Thin Sea Ice Thickness (Snow Depth) Active Microwave Backscatter SAR Sea Ice Classification Sea Ice Motion Very Thin Sea Ice Thickness Scatterometer Sea Ice Type Altimeter Sea Ice Thickness (Snow Depth) Optical Spectral Albedo Spectrometer Floe Sizes Distribution Melt Pond Coverage Melt Pond Depth Infrared Surface Emissivity Radiometer Sea Ice Surface Temperature Thin Sea Ice Thickness Laser Backscatter Altimeter Sea Ice Thickness What are the main processes of sea ice growth & decay? How do they affect sea ice remote sensing? How to observe these processes independently? Formation Redistribution Snow Surface Melt & Growth Processes Annual Cycle of Sea Ice Sea Ice Extent Area covered at least with 15% sea ice (according to passive microwave data) Long-Term Sea Ice Trends Sea Ice Extent Sea Ice Thickness Passive Microwave Time Series Submarines, Moorings, Airborne Surveys Arctic Antarctic No comparable sea ice thickness data record Sea Ice Age & Type Sea Ice Age Sea Ice Type Model with observed ice drift & concentration Passive & Active Microwave Formation Redistribution Snow Surface Melt & Growth Processes Early Sea Ice -

Frazil-Ice Nucleation by Mass-Exchange Processes at the Air- Water Interface
J ournal oIG/acio logy, V o !. 19, No. 8 1, 197 7 FRAZIL-ICE NUCLEATION BY MASS-EXCHANGE PROCESSES AT THE AIR- WATER INTERFACE By T. E. O ST E RKAMP':' (Geophysical Institute, University of Alaska, Fairbanks, A laska 99701, U .S.A. ) AosTRACT. T he physical requirem ents for proposed frazil-ice nucleati on theori es are reviewed in the light of recent observations on frazil-ice forma tion. It is concluded that sponta neous heterogeneous nucleation in a thin supercooled surface la yer of wa ter is not a via ble mechanism for frazil-ice nucleation. E fforts to observe crys ta l multiplicati on b y b order ice have n o t been successful. The mass-excha nge m echanism proposed by O sterka mp and others ( 1974) has been gen era li zed to include splashing, wind spray, bubble bursting, evapo ra tion, a nd ma teria l tha t originates a t a dista nce from the strea m (e. g. snow, frost, ice pa rticles, cold orga nic m a teria l, a nd cold soil pa rticles). I t is shown that these mass-exchange processes ca n account for frazil-ice nucleation under a wide ra nge of phys ical a nd meteorological conditions. It is suggested that secondary nuclea tion may be responsible for large frazil-ice concentra tions in streams and rivers. R EsuME. JVllcieatioll de la glace dll ''.fra zil'' par des processus d'echallges de masses a I'illlerface air ell eall. O n a rev u les conditions physiques requises pa r les theories p our la nucleation d e la glace du " frazil " a la lumicre de recentes observations sur cette forma ti on. -

Snow and Ice Control Around Structures - George D
COLD REGIONS SCIENCE AND MARINE TECHNOLOGY - Snow and Ice Control Around Structures - George D. Ashton SNOW AND ICE CONTROL AROUND STRUCTURES George D. Ashton Consultant, Lebanon, NH 03766 Keywords: ice jams, ice control, flooding, snow drifting, snow loads, river ice Contents 1. Introduction 2. Nature of ice jams 2.1. Frazil Ice 2.1.1. Hanging Dams 2.1.2. Blockage of Intakes 2.2. Breakup Ice Jams 3. Control of ice jams 3.1. Frazil Ice Jams 3.2. Breakup Ice Jams 3.2.1. Ice Suppression 3.2.2. Dikes 3.2.3. Ice Booms 3.2.4. Ice Control Structures 3.2.5. Ice Removal 3.2.6. Ice Breaking 3.2.7. Ice Weakening 3.2.8. Blasting 4. Other Ice Control Techniques 4.1. Air Bubbler Systems 4.1.1. Requirements 4.1.2. Limitations 4.1.3. Operation 4.2. Other Ice Control Techniques 5. Snow control around structures 5.1. Buildings 5.1.1. Snow UNESCOLoads on Roofs – EOLSS 5.1.2. Blowing Snow 5.2. Roads 5.2.1 Snow Fences 6. Conclusion SAMPLE CHAPTERS Glossary Bibliography Biographical Sketch Summary Two main topics are treated here: control of ice jams including mitigation measures, and control of snow accumulations around structures. The nature of ice jams is described and the difference between jams formed of frazil ice and jams formed of broken ice is ©Encyclopedia of Life Support Systems (EOLSS) COLD REGIONS SCIENCE AND MARINE TECHNOLOGY - Snow and Ice Control Around Structures - George D. Ashton discussed. Also discussed are various ice control techniques used for specific problems. -

The Effects of Rotation and Ice Shelf Topography on Frazil-Laden Ice Shelf Water Plumes
The effects of rotation and ice shelf topography on frazil-laden ice shelf water plumes Article Published Version Holland, P. R. and Feltham, D. L. (2006) The effects of rotation and ice shelf topography on frazil-laden ice shelf water plumes. Journal of Physical Oceanography, 36 (12). pp. 2312-2327. ISSN 0022-3670 doi: https://doi.org/10.1175/JPO2970.1 Available at http://centaur.reading.ac.uk/34907/ It is advisable to refer to the publisher’s version if you intend to cite from the work. See Guidance on citing . Published version at: http://dx.doi.org/10.1175/JPO2970.1 To link to this article DOI: http://dx.doi.org/10.1175/JPO2970.1 Publisher: American Meteorological Society All outputs in CentAUR are protected by Intellectual Property Rights law, including copyright law. Copyright and IPR is retained by the creators or other copyright holders. Terms and conditions for use of this material are defined in the End User Agreement . www.reading.ac.uk/centaur CentAUR Central Archive at the University of Reading Reading’s research outputs online 2312 JOURNAL OF PHYSICAL OCEANOGRAPHY VOLUME 36 The Effects of Rotation and Ice Shelf Topography on Frazil-Laden Ice Shelf Water Plumes ϩ PAUL R. HOLLAND* AND DANIEL L. FELTHAM Centre for Polar Observation and Modelling, University College London, London, United Kingdom (Manuscript received 24 June 2005, in final form 20 April 2006) ABSTRACT A model of the dynamics and thermodynamics of a plume of meltwater at the base of an ice shelf is presented. Such ice shelf water plumes may become supercooled and deposit marine ice if they rise (because of the pressure decrease in the in situ freezing temperature), so the model incorporates both melting and freezing at the ice shelf base and a multiple-size-class model of frazil ice dynamics and deposition. -

2Growth, Structure and Properties of Sea
Growth, Structure and Properties 2 of Sea Ice Chris Petrich and Hajo Eicken 2.1 Introduction The substantial reduction in summer Arctic sea ice extent observed in 2007 and 2008 and its potential ecological and geopolitical impacts generated a lot of attention by the media and the general public. The remote-sensing data documenting such recent changes in ice coverage are collected at coarse spatial scales (Chapter 6) and typically cannot resolve details fi ner than about 10 km in lateral extent. However, many of the processes that make sea ice such an important aspect of the polar oceans occur at much smaller scales, ranging from the submillimetre to the metre scale. An understanding of how large-scale behaviour of sea ice monitored by satellite relates to and depends on the processes driving ice growth and decay requires an understanding of the evolution of ice structure and properties at these fi ner scales, and is the subject of this chapter. As demonstrated by many chapters in this book, the macroscopic properties of sea ice are often of most interest in studies of the interaction between sea ice and its environment. They are defi ned as the continuum properties averaged over a specifi c volume (Representative Elementary Volume) or mass of sea ice. The macroscopic properties are determined by the microscopic structure of the ice, i.e. the distribution, size and morphology of ice crystals and inclusions. The challenge is to see both the forest, i.e. the role of sea ice in the environment, and the trees, i.e. the way in which the constituents of sea ice control key properties and processes. -

Laboratory Study of Frazil Ice Accumulation
Discussion Paper | Discussion Paper | Discussion Paper | Discussion Paper | The Cryosphere Discuss., 5, 1835–1886, 2011 The Cryosphere www.the-cryosphere-discuss.net/5/1835/2011/ Discussions TCD doi:10.5194/tcd-5-1835-2011 5, 1835–1886, 2011 © Author(s) 2011. CC Attribution 3.0 License. Laboratory study of This discussion paper is/has been under review for the journal The Cryosphere (TC). frazil ice Please refer to the corresponding final paper in TC if available. accumulation S. De la Rosa and S. Maus Laboratory study of frazil ice Title Page accumulation under wave conditions Abstract Introduction S. De la Rosa1,2 and S. Maus1 Conclusions References 1Geophysical Institute, University of Bergen, Allegaten´ 70, 5007, Bergen, Norway Tables Figures 2Nansen Environmental and Remote Sensing Center/Mohn-Sverdrup Center, Thormøhlensgate 47, 5006, Bergen, Norway J I Received: 27 May 2011 – Accepted: 13 June 2011 – Published: 8 July 2011 J I Correspondence to: S. De la Rosa ([email protected]) Back Close Published by Copernicus Publications on behalf of the European Geosciences Union. Full Screen / Esc Printer-friendly Version Interactive Discussion 1835 Discussion Paper | Discussion Paper | Discussion Paper | Discussion Paper | Abstract TCD Ice growth in turbulent seawater is often accompanied by the accumulation of frazil ice crystals at its surface. The thickness and volume fraction of this ice layer play an 5, 1835–1886, 2011 important role in shaping the gradual transition from a loose to a solid ice cover, how- 5 ever, observations are very sparse. Here we analyse an extensive set of observations Laboratory study of of frazil ice, grown in two parallel tanks with controlled wave conditions and thermal frazil ice forcing, focusing on the first one to two days of grease ice accumulation. -

Chronology, Stable Isotopes, and Glaciochemistry of Perennial Ice in Strickler Cavern, Idaho, USA
Investigation of perennial ice in Strickler Cavern, Idaho, USA Chronology, stable isotopes, and glaciochemistry of perennial ice in Strickler Cavern, Idaho, USA Jeffrey S. Munroe†, Samuel S. O’Keefe, and Andrew L. Gorin Geology Department, Middlebury College, Middlebury, Vermont 05753, USA ABSTRACT INTRODUCTION in successive layers of cave ice can provide a record of past changes in atmospheric circula- Cave ice is an understudied component The past several decades have witnessed a tion (Kern et al., 2011a). Alternating intervals of of the cryosphere that offers potentially sig- massive increase in research attention focused ice accumulation and ablation provide evidence nificant paleoclimate information for mid- on the cryosphere. Work that began in Antarc- of fluctuations in winter snowfall and summer latitude locations. This study investigated tica during the first International Geophysi- temperature over time (e.g., Luetscher et al., a recently discovered cave ice deposit in cal Year in the late 1950s (e.g., Summerhayes, 2005; Stoffel et al., 2009), and changes in cave Strickler Cavern, located in the Lost River 2008), increasingly collaborative efforts to ex- ice mass balances observed through long-term Range of Idaho, United States. Field and tract long ice cores from Antarctica (e.g., Jouzel monitoring have been linked to weather patterns laboratory analyses were combined to de- et al., 2007; Petit et al., 1999) and Greenland (Schöner et al., 2011; Colucci et al., 2016). Pol- termine the origin of the ice, to limit its age, (e.g., Grootes et al., 1993), satellite-based moni- len and other botanical evidence incorporated in to measure and interpret the stable isotope toring of glaciers (e.g., Wahr et al., 2000) and the ice can provide information about changes compositions (O and H) of the ice, and to sea-ice extent (e.g., Serreze et al., 2007), field in surface environments (Feurdean et al., 2011). -

Frazil Ice: a Review of Its Properties, with a Selected Bibliography Williams, G
NRC Publications Archive Archives des publications du CNRC Frazil ice: a review of its properties, with a selected bibliography Williams, G. P. This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur. Publisher’s version / Version de l'éditeur: Engineering Journal, 42, 11, pp. 55-60, 1959-12-01 NRC Publications Record / Notice d'Archives des publications de CNRC: https://nrc-publications.canada.ca/eng/view/object/?id=f683a087-d82c-4542-81e8-398c95993824 https://publications-cnrc.canada.ca/fra/voir/objet/?id=f683a087-d82c-4542-81e8-398c95993824 Access and use of this website and the material on it are subject to the Terms and Conditions set forth at https://nrc-publications.canada.ca/eng/copyright READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE. L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site https://publications-cnrc.canada.ca/fra/droits LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB. Questions? Contact the NRC Publications Archive team at [email protected]. If you wish to email the authors directly, please see the first page of the publication for their contact information. Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. -
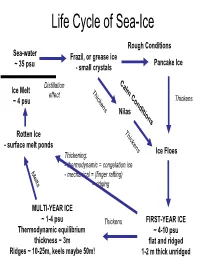
Life Cycle of Sea-Ice
Life Cycle of Sea-Ice Rough Conditions Sea-water Frazil, or grease ice Pancake Ice ~ 35 psu - small crystals C Distillation a Ice Melt T lm effect hi C ~ 4 psu ck o Thickens ens nd Nilas itio ns T hi Rotten Ice c - surface melt ponds kens Ice Floes Thickening: - thermodynamic = congelation ice M e - mechanical = (finger rafting) l ts = ridging MULTI-YEAR ICE ~ 1-4 psu Thickens FIRST-YEAR ICE Thermodynamic equilibrium ~ 4-10 psu thickness ~ 3m flat and ridged Ridges ~ 10-25m, keels maybe 50m! 1-2 m thick unridged Internal Structure of Sea Ice Brine Channels within the ice (~width of human hair) Brine rejected from ice (4-10psu), away from surface, but concentrates in brine channels long crystals as congelation ice (small volume but VERY HIGH SALINITIES) (frozen on from below) -6 deg C -10 deg C -21 deg C 100psu 145psu 216psu Pictures from AWI Brine Volume and Salinity From Thomas and Dieckmann 2002, Science .... adapted from papers by Hajo Eichen Impacts of Sea-ice on the Ocean ICE FORMATION and PRESENCE Wind - brine rejection - Ocean-Atmos momentum barrier - Ocean-Atmos heat barrier - ice edge processes (e.g. upwelling) - keel stirring (i.e. mixing, but < wind) Ocean 10psu MELTING ICE Fresh 35psu - stratification (fresher water) (cf. distillation as ice moves from formation region) Saltier - transport of sediment, etc S increases START FREEZE MELT Impacts of Sea-ice on the Atmosphere ICE PRESENCE - albedo change - Ocean-Atmos momentum barrier - Ocean-Atmos heat barrier Water Sky Sea Smoke Heat balance S=Shortwave radiation from sun (reflects off clouds and surface) albedo= how much radiation reflects from surface albedo of ice ~ 0.8 albedo of water ~ 0.04 (if sun overhead) L=Longwave radiation (from surface and clouds) F=Heat flux from Ocean M=Melt (snow and ice) From N. -

Prevention of Frazil Ice Clogging of Water Intakes by Application of Heat
REC-ERC-74-15 PREVENTION OF FRAZIL ICE CLOGGING OF WATER INTAKES BY APPLICATION OF HEAT Engineering and Research Center Bureau of Reclamation September 1974 Prepared for ICE RESEARCH MANAGEMENT COMMITTEE MS-230 (8-70) Bureau of R·~clamation TITLE PAGE 1. REPORT NO. REC-EF:C-74-15 4. TITLE: AND SUBTITLE 5. REPORT DATE September 1974 Prevention of Frazil Ice Clogging of Water 6. PERFORMING ORGANIZATION CODE Intakes by Application of Heat 7. AUTHOR(S) 8. PERFORMING ORGANIZATION REPORT NO. T. H. Logan REC-ERC-74-15 9. PERFORMING ORGANIZATION NAME AND ADDRESS 10. WORK UNIT NO. Bureau of Reclamation 11. CONTRACT OR GRANT NO. Engineeliing and Research Center Denver, Colorado 80225 13. TYPE OF REPORT AND PERIOD COVERED . SPONSORING AGENCY NAME AND 14. SPONSORING AGENCY CODE 15. SUPPLEMENTARY NOTES 16. ABSTRACT The phenomenon of ice formation in flowing water and the technology of heating trashrack bars to prevent clogging by frazil ice are reviewed. The report includes: (1) A description of frazil ice formation, (2) development of heat transfer equations for trashrack bars immersed in a fluid, (3) correlation between conditions assumed in developing the theoretical expressions and actual conditions present in a water intake, (4) economics of heating trash rack bars, (5) methods of heating trash rack bars, and (6) recommendations for future studies. Has 64 references. 17. KEY WORDS AND DOCUMENT ANALYSIS a. DESCRIPTORS-- I *frazil ice/ ice/ canals/ floating ice/ ice cover/ open channels/ slush/ intake structures/ *heatin~)/ trashracks/ barriers/ bibliographies b. IDENT/F /ERS-- c. COSATI Field/Group 13M 1. NO. OF PAGE Available from the National Technical Information Service, Operations 20 Division, Springfield, Virginia 22151.