Zootaxa, Description of Halichoeres Rubrovirens, a New Species Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Halichoeres Bivittatus (Bloch, 1791) Frequent Synonyms / Misidentifications: None / Halichoeres Maculipinna (Müller and Troschel, 1848)
click for previous page 1710 Bony Fishes Halichoeres bivittatus (Bloch, 1791) Frequent synonyms / misidentifications: None / Halichoeres maculipinna (Müller and Troschel, 1848). FAO names: En - Slippery dick. Diagnostic characters: Body slender, depth 3.3 to 4.6 in standard length.Head rounded and scaleless;snout blunt; 1 pair of enlarged canine teeth at front of upper jaw and a small canine posteriorly near corner of mouth; 2 pairs of enlarged canine teeth anteriorly in lower jaw. Gill rakers on first arch 16 to 19. Dorsal fin continu- ous, with 9 spines and 11 soft rays;anal fin with 3 spines and 9 soft rays;caudal fin rounded;pectoral-fin rays 13. Lateral line continuous with an abrupt downward bend beneath soft portion of dorsal fin, and 27 pored scales. Colour: body colour variable, primarily pale green to white ground colour with a dark midbody stripe, a second lower stripe often present but less distinct; small green and yellow bicoloured spot above pectoral fin; pinkish or orange markings on the head, these sometimes outlined with pale blue; in adults, the tips of the cau- dal-fin lobes are black. Size: Maximum length to about 20 cm. Habitat, biology, and fisheries: Inhabits a di- versity of habitats from coral reef to rocky reef and seagrass beds. Any disturbance of the bot- tom, such as the overturning of a rock will attract a swarm of them, all hoping to find food uncov- ered. Feeds omnivorously on crabs, fishes, sea urchins, polychaetes, molluscs, and brittle stars. This species is not marketed for food, but is com- monly seen in the aquarium trade. -
Download the Full List of Species Name Changes For
Fishes of the Maldives Indian Ocean 2014 Photo Guide to Fishes of the Maldives 1998 Family Scientific Name Common Name Page Scientific Name Changed Common Name Changed Page Solderfishes Holocentridae-2 Myripristis melanosticta Splendid Soldierfish 78 Myripristis botche 47 Ghost Pipefishes Solenostomidae Solenostomus halimeda Coralline Ghost Pipefish 81 Solenostome sp. 1 50 Pipefishes Syngnathidae Corythoichthys haematopterus Reef-top Pipefish 82 Corythoichtys haematopterus* 51 Corythoichthys schultzi Schultz’s Pipefish 83 Corythoichtys schultzi* 51 Corythoichthys flavofasciatus Yellow-banded Pipefish 83 Corythoichtys flavofasciatus* 51 Corythoichthys insularis Cheeked Pipefish 83 Corythoichtys insularis* 51 Doryrhamphus excisus Blue-stripe Pipefish 84 Doryrhamphus exicus* 53 Hippocampus jayakari Spiny Seahorse 85 Hippocampus hystrix 53 Scorpionfishes Scorpaenidae-2 Scorpaenopsis diabolus False Stonefish 89 Scorpaenopsis diabola 57 Groupers Serranidae-2 Cephalopholis leopardus Leopard Rock Cod 98 Cephalopholis leoparda 66 Basslets Serranidae-3 Pseudanthias lunulatus Yellow-eye Basslet 107 Pseudanthias n. sp. 74 Pseudanthias bimarginatus Short-snout Basslet 109 Pseudanthias parvirostris 76 Cardinalfishes Apogonidae Cheilodipterus lineatus Tiger Cardinalfish 114 Cheilodipterus macrodon 81 Apogon urostigma Spiny-head Cardinalfish 117 Apogon kallopterus 83 Apogon melanorhynchus Spiny-eye Cardinalfish 117 Apogon fraenatus 84 Apogon fraenatus Tapered-line Cardinalfish 117 Apogon exostigma 84 Apogon savayensis Big-eye Dusky-cardinalfish 118 Apogon -

Fishery Conservation and Management Pt. 622, App. A
Fishery Conservation and Management Pt. 622, App. A vessel's unsorted catch of Gulf reef to complete prohibition), and seasonal fish: or area closures. (1) The requirement for a valid com- (g) South Atlantic golden crab. MSY, mercial vessel permit for Gulf reef fish ABC, TAC, quotas (including quotas in order to sell Gulf reef fish. equal to zero), trip limits, minimum (2) Minimum size limits for Gulf reef sizes, gear regulations and restrictions, fish. permit requirements, seasonal or area (3) Bag limits for Gulf reef fish. closures, time frame for recovery of (4) The prohibition on sale of Gulf golden crab if overfished, fishing year reef fish after a quota closure. (adjustment not to exceed 2 months), (b) Other provisions of this part not- observer requirements, and authority withstanding, a dealer in a Gulf state for the RD to close the fishery when a is exempt from the requirement for a quota is reached or is projected to be dealer permit for Gulf reef fish to re- reached. ceive Gulf reef fish harvested from the (h) South Atlantic shrimp. Certified Gulf EEZ by a vessel in the Gulf BRDs and BRD specifications. groundfish trawl fishery. [61 FR 34934, July 3, 1996, as amended at 61 FR 43960, Aug. 27, 1996; 62 FR 13988, Mar. 25, § 622.48 Adjustment of management 1997; 62 FR 18539, Apr. 16, 1997] measures. In accordance with the framework APPENDIX A TO PART 622ÐSPECIES procedures of the applicable FMPs, the TABLES RD may establish or modify the follow- TABLE 1 OF APPENDIX A TO PART 622Ð ing management measures: CARIBBEAN CORAL REEF RESOURCES (a) Caribbean coral reef resources. -

Phylogenetics and Geography of Speciation in New World Halichoeres T Wrasses ⁎ Peter C
Molecular Phylogenetics and Evolution 121 (2018) 35–45 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev Phylogenetics and geography of speciation in New World Halichoeres T wrasses ⁎ Peter C. Wainwrighta, , Francesco Santinib, David R. Bellwoodc, D. Ross Robertsond, Luiz A. Rochae, Michael E. Alfarob a Department of Evolution and Ecology, University of California, Davis, One Shields Avenue, Davis, CA 95616, USA b University of California Los Angeles, Department of Ecology and Evolutionary Biology, 610 Charles E Young Drive South, Los Angeles, CA 90095, USA c Centre of Excellence for Coral Reef Studies, James Cook University, Townsville, QLD, 4811, Australia d Smithsonian Tropical Research Institute, Balboa, Rep. de Panama, Unit 0948, APO AA 34002, USA e California Academy of Sciences, Section of Ichthyology, 55 Music Concourse Drive, San Francisco, CA 94118, USA ARTICLE INFO ABSTRACT Keywords: The New World Halichoeres comprises about 30 small to medium sized wrasse species that are prominent Labridae members of reef communities throughout the tropical Western Atlantic and Eastern Pacific. We conducted a New World Halichoeres phylogenetic analysis of this group and related lineages using new and previously published sequence data. We Western Atlantic estimated divergence times, evaluated the monophyly of this group, their relationship to other labrids, as well as Eastern Pacific the time-course and geography of speciation. These analyses show that all members of New World Halichoeres Phylogeny form a monophyletic group that includes Oxyjulis and Sagittalarva. New World Halichoeres is one of numerous Biogeography Speciation labrid groups that appear to have radiated rapidly about 32 Ma and form a large polytomy within the julidine wrasses. -

Chec List an Update to the List of Coral Reef Fishes from Koh Tao, Gulf Of
Check List 10(5): 1123–1133, 2014 © 2014 Check List and Authors Chec List ISSN 1809-127X (available at www.checklist.org.br) Journal of species lists and distribution An update to the list PECIES S Gulf of Thailand OF of coral reef fishes from Koh Tao, 1* 2 ISTS Patrick Scaps and Chad Scott L 1 Laboratoire de Biologie animale,[email protected] Université des Sciences et Technologies de Lille, 59 655 Villeneuve d’Ascq Cédex, France. 2 New Heaven Reef Conservation Program, 48 Moo 3, Koh Tao, Suratthani, Thailand, 84360. * Corresponding author: E-mail: ABSTRACT: (i.e., cryptic species or transient Twenty-one species are reported for the first time from Koh Tao (Turtle Island) in the Gulf of Thailand. TInformationhis and photographs were obtained from local scuba divers in order to censusAntennatus rare and Histrio), Ophichthyidae (genusspecies onlyCallechelys present), duringPlatycephalidae one season) (genus or not previouslyThysanophrys recorded), Plotosidae fish species (genus living Plotosus on or near) and coral Synanceiidae reefs from the(genera area. Inimicus is the and first Synanceia time that species belonging to the families AntennariidaePseudobalistes, (genera Balistidae; Cyclichthys, Diodontidae; Bolbometopon, Scaridae; and Hippocampus, ), and reef-fish genera of severalAntennatus families nummifer ( (Antennariidae), Pseudobalistes marginatus (Balistidae), Monacanthus chinensis (Monacanthidae),Syngnathidae), Callechelys among others,marmora haveta been(Ophichthyidae), recorded in KohThysnophrys Tao. Of the cf. 21 chiltonae species reported(Platycephalidae), for the first Bolbometopon time from muricatumKoh Tao, 7 (Scaridae) species ( and Synanceia cf. verrucosa (Synanceidae)) are new records for the species found in the present study. Gulf of Thailand. To date, 223 species of coral reef fishes belonging to 53 families are known from Koh Tao, including the 10.15560/10.5.1123 DOI: Introduction MaterialS and Methods 2 archipelago in the western Gulf of Thailand. -

Shallow Water Records of Fish
Shallow water records of fish Collected from or observed at Howland Island from 1927-2002. Collected or compiled by Mundy et al. (2002). Scientific Name Common Name GINGLYMOSTOMATIDAE Nurse Sharks Nebrius ferrugineus nurse shark CARCHARHINIDAE Requiem Sharks Carcharhinus amblyrhynchos grey reef shark Carcharhinus melanopterus reef blacktip shark Galeocerdo cuvieri tiger shark HEMIGALEIDAE Weasel Sharks Triaenodon obesus reef whitetip shark SPHYRNIDAE Hammerhead Sharks Sphyrna lewini scalloped hammerhead shark DASYATIDAE Sand Rays Taeniura meyeni MYLIOBATIDIDAE Eagle Rays Aetobatus narinari spotted eagle ray MOBULIDAE Manta Rays Manta sp. manta MURAENIDAE Moray Eels Anarchias allardicei Allardice’s moray Anarchias cantonenesis Canton Island moray Echidna nebulosa snowflake moray Echidna polyzona barred moray Enchelycore pardalis Gymnomuraena zebra zebra moray Gymnothorax breedini Gymnothorax chilospilus Gymnothorax javanicus giant moray Gymnothorax flavimarginatus yellow-margined moray Gymnothorax marshallensis Marshall Island moray Gymnothorax meleagris white-mouth moray Gymnothorax picta peppered moray Gymnothorax rueppelliae yellow-headed moray Gymnothorax sp. Gymnothorax thyrsoideus Gymnothorax undulatus undulated moray Uropterygius sp. Uropterygius marmoratus marbled snake moray OPHICHTHIDAE Myrichthys maculosus spotted snake eel CONGRIDAE Conger Eel Conger sp. CHANIDAE Milkfish Chanos chanos milkfish SYNODONTIDAE Lizardfishes Synodus sp. HOLOCENTRIDAE Squirrelfishes Myripristis berndti bigscale soldierfish Sargocentron caudimaculatum -
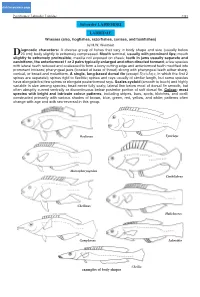
Suborder LABROIDEI LABRIDAE
click for previous page Perciformes: Labroidei: Labridae 3381 Suborder LABROIDEI LABRIDAE Wrasses (also, hogfishes, razorfishes, corises, and tuskfishes) by M.W. Westneat iagnostic characters: A diverse group of fishes that vary in body shape and size (usually below D20 cm); body slightly to extremely compressed. Mouth terminal, usually with prominent lips; mouth slightly to extremely protrusible; maxilla not exposed on cheek; teeth in jaws usually separate and caniniform, the anteriormost 1 or 2 pairs typically enlarged and often directed forward; a few species with lateral teeth reduced and coalesced to form a bony cutting edge and anteriormost teeth modified into prominent incisors; pharyngeal jaws (located at base of throat) strong with pharyngeal teeth either sharp, conical, or broad and molariform. A single, long-based dorsal fin (except Xyrichtys, in which the first 2 spines are separate); spines rigid to flexible; spines and rays usually of similar length, but some species have elongate first few spines or elongate posteriormost rays. Scales cycloid (smooth to touch) and highly variable in size among species; head never fully scaly; lateral line below most of dorsal fin smooth, but often abruptly curved ventrally or discontinuous below posterior portion of soft dorsal fin. Colour: most species with bright and intricate colour patterns, including stripes, bars, spots, blotches, and ocelli constructed primarily with various shades of brown, blue, green, red, yellow, and white; patterns often change with age and with sex-reversal in this group. Bodianus Xyrichtys Macropharyngodon Cirrhilabrus Cheilinus Halichoeres Gomphosus Labroides Cheilio examples of body shapes 3382 Bony Fishes Habitat, biology, and fisheries: Labrids are most common in shallow waters in a variety of habitats such as coral reefs, rocky reefs, sand, grass, and algae. -

NOAA Technical Report NMFS SSRF-781
781 NOAA Technical Report NMFS SSRF-781 .<°:x An Annotated Checklist of the Fishes of Samoa Richard C. Wass May 1984 Marine Biological I Laboratory | LIBRARY j OCT 14 1992 ! Woods Hole, Mass U.S. DEPARTMENT OF COMMERCE National Oceanic and Atmospheric Adnninistration National Marine Fisheries Service . NOAA TECHNICAL REPORTS National Marine Fisheries Service, Special Scientific Report—Fisheries The major responsibilities of the National Marine Fisheries Service (NMFS) are to monitor and assess the abundance and geographic distribution of fishery resources, to understand and predict fluctuations in the quantity and distribution of these resources, and to establish levels for optimum use of the enforcement resources. NMFS is also charged with the development and implementation of policies for managing national fishing grounds, development and of domestic fisheries regulations, surveillance of foreign fishing off United States coastal waters, and the development and enforcement of international fishery agreements and policies. NMFS also assists the fishing industry through marketing service and economic analysis programs, and mortgage insurance and vessel construction subsidies. It collects, analyzes, and publishes statistics on various phases of the industry. The Special Scientific Report— Fisheries series was established in 1949. The series carries reports on scientific investigations that document long-term continuing programs of NMFS, or intensive scientific reports on studies of restricted scope. The reports may deal with applied fishery problems. The series is also used as a medium for the publication of bibhographies of a specialized scientific nature. NOAA Technical Repons NMFS SSRF are available free in limited numbers to governmental agencies, both Federal and State. They are also available in exchange for other scientific and technical publications in the marine sciences. -

Checklist of the Species of the Families Labridae and Scaridae: an Update
PARENTI & RANDALL Checklist of Labridae and Scaridae 29 Checklist of the species of the families Labridae and Scaridae: an update Paolo Parenti1 and John E. Randall2 1University of Milano–Bicocca, Department of Environmental Sciences, Piazza della Scienza, 1, 20126 Milano, Italy. Email: [email protected] 2Bishop Museum, 1525 Bernice St., Honolulu, HI 96817–2704, USA Email: [email protected] Recieved 7 July, accepted 1 October 2010 ABSTRACT. The checklist of the species of the RÉSUMÉ. Le répertoire des espèces des familles families Labridae and Scaridae is updated. des Labridae et des Scaridae est mis à jour. Après After the publication of the annotated checklist la publication du répertoire annoté (Parenti & (Parenti & Randall 2000) the species of Labridae Randall, 2000), les espèces Labridae ont augmenté increased from 453 to 504 and genera from 68 to 70, de 453 à 504 et les genres de 68 à 70, tandis que les whereas Scaridae increased from 88 to 99 species. Scaridae sont passées de 88 à 99 espèces. Altogether this account lists 53 species that are new Dans l’ensemble, ce comptage dénombre 53 espèces to science, while 14 species have been resurrected qui sont nouvelles à la science, tandis que 14 ont été from synonymy and 5 are now regarded as junior ressuscitées des synonymes et 5 sont actuellement synonyms. Comments on the status of 20 nominal considérées sous 5 nouveaux synonymes. Le species and notes on undescribed species are also répertoire comprend également des commentaires included. sur l’état de 20 espèces et des notes d’explication des espèces non encore décrites. -

Derek FL.Xlsx
2017-12-05 Fish Common Name Scientific Name Retail Price Chromis/Damsels Red Chromis Chromis sp. $ - Green Chromis Chromis viridis $ 10.00 Yellow Tail Damsel Chrysiptera parasema $ 10.00 Talbots Damsel Chrysiptera talboti $ 10.00 Anthia Carberryi Anthias F Nemanthias carberryi $ 32.00 Carberryi Anthias M Nemanthias carberryi $ 48.00 Redbar Anthia Pseudanthias cooperi $ 52.00 Evansi Anthia Pseudanthias evansi $ 54.00 Square Back Anthia L - Female Pseudanthias pleurotaenia $ 48.00 Square Back Anthia L - Male Pseudanthias pleurotaenia $ 84.00 Princess Anthia M Pseudanthias smithvanizi $ 60.00 Lyretail Anthia F Pseudanthias squamipinnis $ 31.00 Lyretail Anthia M Pseudanthias squamipinnis $ 40.00 Purple Queen Anthia Pseudanthias tuka $ 40.00 Fat Head Anthia Serranocirrhites latus $ 110.00 Angels Flagfin Angel T Apolemichthys trimaculatus $ 50.00 Flameback Angel S Centropyge acanthops $ 80.00 Pygmy Angelfish - Atlantic Centropyge argi $ 56.00 BiColour Angel S <2.5" Centropyge bicolor $ 40.00 BiColour Angel M <3.5" Centropyge bicolor $ 50.00 Coral Beauty Angel M - SALE Centropyge bispinosa $ 50.00 Hawaii Lemon Peel Angel Centropyge flavissima $ 80.00 Yellow Angel Centropyge heraldi $ 44.00 Hawaii Flame Angel Centropyge loricula $ 104.00 Keyhole Angel Centropyge tibicen $ 44.00 Aussie - True Personifer Angel M Chaetodontoplus personifer $ 900.00 Bellus Angel - Female Large Genicanthus bellus (F) $ 190.00 Spotbreast Lyretail Angel F Med Genicanthus melanospilos $ 90.00 African Angel Holacanthus africanus $ - Emperor Angel - Juvi S Pomacanthus -

Lyretail Anthias
SECOND QUARTER 2018 I VOLUME 12 BREEDING & REARING LYRETAIL ANTHIAS HUNTING ZOA-EATING NUDIBRANCHS SOFT CORAL FRAGGING GUIDE REEF SPOTLIGHTS: MEXICO CITY REEF, SHROOM LAGOON & CUNHA REEF Reef Hobbyist Magazine 1 SECOND QUARTER 2018 | Volume 12 FEATURES Copyright © 2018 Reef Hobbyist Magazine. All rights reserved. MEXICO CITY REEF ANNOUNCEMENTS Andres Corral is a veteran reefkeeper 6 who believes that reef tanks are • Wish there was a freshwater magazine like RHM? Now there is! Aquarium ambassadors for wild reefs, which many people rarely Hobbyist Magazine is now available for FREE in the best local fish stores get to see. Here, he shares his personal 250-gallon around the country and online at www.aquariumhobbyistmagazine.com! mixed reef aquarium. • Care to share your reefing, fragging, breeding, or husbandry success with the world? Email us your article ideas through the "Contact Us" tab on our THE INTERRUPTUS ANGEL: website. A CENTROPYGE CENTERPIECE 12 Colby Podkin-Johnson is an all-around fish nerd and the owner of Pacific Island Aquatics. Learn how RHM-SPONSORED EVENTS to choose and care for this coveted and beautiful pygmy • Reef-A-Palooza (FL): April 7–8, Orlando, FL – www.reefapaloozashow.net angelfish. • ReefSMART: April 21, Raleigh, NC – www.sustainablereef.com SHROOM LAGOON • LMAR Frag Swap: April 29, San Antonio, TX – www.maast.org Darwin Ngo is a co-founder of Legendary • Ladies Frag Swapping: May 12, Sturgis, MI – www.ladiesfragswapping.weebly.com 18 Corals and lives in San Jose, CA. After • Florida Frag Swap: June 2, Hialeah, FL – www.flfragswap.com transitioning through a couple of different nanos, Darwin • Reef-A-Palooza (NY): June 23–24, Secaucus, NJ – www.reefapaloozashow.net finally found one that was just right. -
Checklist of the Tidal Pool Fishes of Jeju Island, Korea
A peer-reviewed open-access journal ZooKeys 709: 135–154 (2017) Checklist of the tidal pool fishes of Jeju Island, Korea 135 doi: 10.3897/zookeys.709.14711 CHECKLIST http://zookeys.pensoft.net Launched to accelerate biodiversity research Checklist of the tidal pool fishes of Jeju Island, Korea Hyuck Joon Kwun1, Jinsoon Park2, Hye Seon Kim1, Ju-Hee Kim1, Hyo-Seon Park1 1 National Marine Biodiversity Institute of Korea, 75, 101 Jangsan-ro, Janghang-eup, Seocheon-gun, Chungcheongnam-do 33662, Korea 2 Korea Maritime and Ocean University, 727 Taejong-ro, Yeongdo-gu, Busan 49112, Korea Corresponding author: Hyuck Joon Kwun ([email protected]) Academic editor: S. Kullander | Received 26 June 2017 | Accepted 14 September 2017 | Published 18 October 2017 http://zoobank.org/9D7FBBFE-998B-4ED3-8D0D-7DB579E442D2 Citation: Kwun HJ, Park J, Kim HS, Kim J-H, Park H-S (2017) Checklist of the tidal pool fishes of Jeju Island, Korea. ZooKeys 709: 135–154. https://doi.org/10.3897/zookeys.709.14711 Abstract Seventy-six species of fishes, representing 60 genera and 34 families, were recorded from tidal pools on Jeju Island, southern Korea. The major families in terms of species were the Gobiidae (11 species), Poma- centridae (8 species), Blenniidae (6 species), and Labridae (5 species). Thirty-nine species were classified as tropical, 26 as temperate and 11 as subtropical. Keywords coastal habitats, fish diversity, inventory, northwestern Pacific Introduction Jeju Island is located southwest of the Korean Peninsula (Kim and Go 2003, Kim et al. 2009, Kwun et al. 2017) and has a volcanic rocky shoreline. The island lies in the southernmost temperate region of Korea, and many subtropical and temperate species of fishes inhabit the coastal and adjacent waters of the island (Kim 2009).