Computational Geology 2 -- Speaking Logarithmically
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

18.01A Topic 5: Second Fundamental Theorem, Lnx As an Integral. Read
18.01A Topic 5: Second fundamental theorem, ln x as an integral. Read: SN: PI, FT. 0 R b b First Fundamental Theorem: F = f ⇒ a f(x) dx = F (x)|a Questions: 1. Why dx? 2. Given f(x) does F (x) always exist? Answer to question 1. Pn R b a) Riemann sum: Area ≈ 1 f(ci)∆x → a f(x) dx In the limit the sum becomes an integral and the finite ∆x becomes the infinitesimal dx. b) The dx helps with change of variable. a b 1 Example: Compute R √ 1 dx. 0 1−x2 Let x = sin u. dx du = cos u ⇒ dx = cos u du, x = 0 ⇒ u = 0, x = 1 ⇒ u = π/2. 1 π/2 π/2 π/2 R √ 1 R √ 1 R cos u R Substituting: 2 dx = cos u du = du = du = π/2. 0 1−x 0 1−sin2 u 0 cos u 0 Answer to question 2. Yes → Second Fundamental Theorem: Z x If f is continuous and F (x) = f(u) du then F 0(x) = f(x). a I.e. f always has an anit-derivative. This is subtle: we have defined a NEW function F (x) using the definite integral. Note we needed a dummy variable u for integration because x was already taken. Z x+∆x f(x) proof: ∆area = f(x) dx 44 4 x Z x+∆x Z x o area = ∆F = f(x) dx − f(x) dx 0 0 = F (x + ∆x) − F (x) = ∆F. x x + ∆x ∆F But, also ∆area ≈ f(x) ∆x ⇒ ∆F ≈ f(x) ∆x or ≈ f(x). -

The Enigmatic Number E: a History in Verse and Its Uses in the Mathematics Classroom
To appear in MAA Loci: Convergence The Enigmatic Number e: A History in Verse and Its Uses in the Mathematics Classroom Sarah Glaz Department of Mathematics University of Connecticut Storrs, CT 06269 [email protected] Introduction In this article we present a history of e in verse—an annotated poem: The Enigmatic Number e . The annotation consists of hyperlinks leading to biographies of the mathematicians appearing in the poem, and to explanations of the mathematical notions and ideas presented in the poem. The intention is to celebrate the history of this venerable number in verse, and to put the mathematical ideas connected with it in historical and artistic context. The poem may also be used by educators in any mathematics course in which the number e appears, and those are as varied as e's multifaceted history. The sections following the poem provide suggestions and resources for the use of the poem as a pedagogical tool in a variety of mathematics courses. They also place these suggestions in the context of other efforts made by educators in this direction by briefly outlining the uses of historical mathematical poems for teaching mathematics at high-school and college level. Historical Background The number e is a newcomer to the mathematical pantheon of numbers denoted by letters: it made several indirect appearances in the 17 th and 18 th centuries, and acquired its letter designation only in 1731. Our history of e starts with John Napier (1550-1617) who defined logarithms through a process called dynamical analogy [1]. Napier aimed to simplify multiplication (and in the same time also simplify division and exponentiation), by finding a model which transforms multiplication into addition. -

TI 83/84: Scientific Notation on Your Calculator
TI 83/84: Scientific Notation on your calculator this is above the comma, next to the square root! choose the proper MODE : Normal or Sci entering numbers: 2.39 x 106 on calculator: 2.39 2nd EE 6 ENTER reading numbers: 2.39E6 on the calculator means for us. • When you're in Normal mode, the calculator will write regular numbers unless they get too big or too small, when it will switch to scientific notation. • In Sci mode, the calculator displays every answer as scientific notation. • In both modes, you can type in numbers in scientific notation or as regular numbers. Humans should never, ever, ever write scientific notation using the calculator’s E notation! Try these problems. Answer in scientific notation, and round decimals to two places. 2.39 x 1016 (5) 2.39 x 109+ 4.7 x 10 10 (4) 4.7 x 10−3 3.01 103 1.07 10 0 2.39 10 5 4.94 10 10 5.09 1018 − 3.76 10−− 1 2.39 10 5 7.93 10 8 Remember to change your MODE back to Normal when you're done. Using the STORE key: Let's say that you want to store a number so that you can use it later, or that you want to store answers for several different variables, and then use them together in one problem. Here's how: Enter the number, then press STO (above the ON key), then press ALPHA and the letter you want. (The letters are in alphabetical order above the other keys.) Then press ENTER. -

Primitive Number Types
Primitive number types Values of each of the primitive number types Java has these 4 primitive integral types: byte: A value occupies 1 byte (8 bits). The range of values is -2^7..2^7-1, or -128..127 short: A value occupies 2 bytes (16 bits). The range of values is -2^15..2^15-1 int: A value occupies 4 bytes (32 bits). The range of values is -2^31..2^31-1 long: A value occupies 8 bytes (64 bits). The range of values is -2^63..2^63-1 and two “floating-point” types, whose values are approximations to the real numbers: float: A value occupies 4 bytes (32 bits). double: A value occupies 8 bytes (64 bits). Values of the integral types are maintained in two’s complement notation (see the dictionary entry for two’s complement notation). A discussion of floating-point values is outside the scope of this website, except to say that some bits are used for the mantissa and some for the exponent and that infinity and NaN (not a number) are both floating-point values. We don’t discuss this further. Generally, one uses mainly types int and double. But if you are declaring a large array and you know that the values fit in a byte, you can save ¾ of the space using a byte array instead of an int array, e.g. byte[] b= new byte[1000]; Operations on the primitive integer types Types byte and short have no operations. Instead, operations on their values int and long operations are treated as if the values were of type int. -

Applications of the Exponential and Natural Logarithm Functions
M06_GOLD7774_14_SE_C05.indd Page 254 09/11/16 7:31 PM localadmin /202/AW00221/9780134437774_GOLDSTEIN/GOLDSTEIN_CALCULUS_AND_ITS_APPLICATIONS_14E1 ... FOR REVIEW BY POTENTIAL ADOPTERS ONLY chapter 5SAMPLE Applications of the Exponential and Natural Logarithm Functions 5.1 Exponential Growth and Decay 5.4 Further Exponential Models 5.2 Compound Interest 5.3 Applications of the Natural Logarithm Function to Economics n Chapter 4, we introduced the exponential function y = ex and the natural logarithm Ifunction y = ln x, and we studied their most important properties. It is by no means clear that these functions have any substantial connection with the physical world. How- ever, as this chapter will demonstrate, the exponential and natural logarithm functions are involved in the study of many physical problems, often in a very curious and unex- pected way. 5.1 Exponential Growth and Decay Exponential Growth You walk into your kitchen one day and you notice that the overripe bananas that you left on the counter invited unwanted guests: fruit flies. To take advantage of this pesky situation, you decide to study the growth of the fruit flies colony. It didn’t take you too FOR REVIEW long to make your first observation: The colony is increasing at a rate that is propor- tional to its size. That is, the more fruit flies, the faster their number grows. “The derivative is a rate To help us model this population growth, we introduce some notation. Let P(t) of change.” See Sec. 1.7, denote the number of fruit flies in your kitchen, t days from the moment you first p. -

C++ Data Types
Software Design & Programming I Starting Out with C++ (From Control Structures through Objects) 7th Edition Written by: Tony Gaddis Pearson - Addison Wesley ISBN: 13-978-0-132-57625-3 Chapter 2 (Part II) Introduction to C++ The char Data Type (Sample Program) Character and String Constants The char Data Type Program 2-12 assigns character constants to the variable letter. Anytime a program works with a character, it internally works with the code used to represent that character, so this program is still assigning the values 65 and 66 to letter. Character constants can only hold a single character. To store a series of characters in a constant we need a string constant. In the following example, 'H' is a character constant and "Hello" is a string constant. Notice that a character constant is enclosed in single quotation marks whereas a string constant is enclosed in double quotation marks. cout << ‘H’ << endl; cout << “Hello” << endl; The char Data Type Strings, which allow a series of characters to be stored in consecutive memory locations, can be virtually any length. This means that there must be some way for the program to know how long the string is. In C++ this is done by appending an extra byte to the end of string constants. In this last byte, the number 0 is stored. It is called the null terminator or null character and marks the end of the string. Don’t confuse the null terminator with the character '0'. If you look at Appendix A you will see that the character '0' has ASCII code 48, whereas the null terminator has ASCII code 0. -

Floating Point Numbers
Floating Point Numbers CS031 September 12, 2011 Motivation We’ve seen how unsigned and signed integers are represented by a computer. We’d like to represent decimal numbers like 3.7510 as well. By learning how these numbers are represented in hardware, we can understand and avoid pitfalls of using them in our code. Fixed-Point Binary Representation Fractional numbers are represented in binary much like integers are, but negative exponents and a decimal point are used. 3.7510 = 2+1+0.5+0.25 1 0 -1 -2 = 2 +2 +2 +2 = 11.112 Not all numbers have a finite representation: 0.110 = 0.0625+0.03125+0.0078125+… -4 -5 -8 -9 = 2 +2 +2 +2 +… = 0.00011001100110011… 2 Computer Representation Goals Fixed-point representation assumes no space limitations, so it’s infeasible here. Assume we have 32 bits per number. We want to represent as many decimal numbers as we can, don’t want to sacrifice range. Adding two numbers should be as similar to adding signed integers as possible. Comparing two numbers should be straightforward and intuitive in this representation. The Challenges How many distinct numbers can we represent with 32 bits? Answer: 232 We must decide which numbers to represent. Suppose we want to represent both 3.7510 = 11.112 and 7.510 = 111.12. How will the computer distinguish between them in our representation? Excursus – Scientific Notation 913.8 = 91.38 x 101 = 9.138 x 102 = 0.9138 x 103 We call the final 3 representations scientific notation. It’s standard to use the format 9.138 x 102 (exactly one non-zero digit before the decimal point) for a unique representation. -

Floating Point Numbers and Arithmetic
Overview Floating Point Numbers & • Floating Point Numbers Arithmetic • Motivation: Decimal Scientific Notation – Binary Scientific Notation • Floating Point Representation inside computer (binary) – Greater range, precision • Decimal to Floating Point conversion, and vice versa • Big Idea: Type is not associated with data • MIPS floating point instructions, registers CS 160 Ward 1 CS 160 Ward 2 Review of Numbers Other Numbers •What about other numbers? • Computers are made to deal with –Very large numbers? (seconds/century) numbers 9 3,155,760,00010 (3.1557610 x 10 ) • What can we represent in N bits? –Very small numbers? (atomic diameter) -8 – Unsigned integers: 0.0000000110 (1.010 x 10 ) 0to2N -1 –Rationals (repeating pattern) 2/3 (0.666666666. .) – Signed Integers (Two’s Complement) (N-1) (N-1) –Irrationals -2 to 2 -1 21/2 (1.414213562373. .) –Transcendentals e (2.718...), π (3.141...) •All represented in scientific notation CS 160 Ward 3 CS 160 Ward 4 Scientific Notation Review Scientific Notation for Binary Numbers mantissa exponent Mantissa exponent 23 -1 6.02 x 10 1.0two x 2 decimal point radix (base) “binary point” radix (base) •Computer arithmetic that supports it called •Normalized form: no leadings 0s floating point, because it represents (exactly one digit to left of decimal point) numbers where binary point is not fixed, as it is for integers •Alternatives to representing 1/1,000,000,000 –Declare such variable in C as float –Normalized: 1.0 x 10-9 –Not normalized: 0.1 x 10-8, 10.0 x 10-10 CS 160 Ward 5 CS 160 Ward 6 Floating -
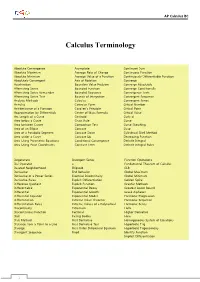
Calculus Terminology
AP Calculus BC Calculus Terminology Absolute Convergence Asymptote Continued Sum Absolute Maximum Average Rate of Change Continuous Function Absolute Minimum Average Value of a Function Continuously Differentiable Function Absolutely Convergent Axis of Rotation Converge Acceleration Boundary Value Problem Converge Absolutely Alternating Series Bounded Function Converge Conditionally Alternating Series Remainder Bounded Sequence Convergence Tests Alternating Series Test Bounds of Integration Convergent Sequence Analytic Methods Calculus Convergent Series Annulus Cartesian Form Critical Number Antiderivative of a Function Cavalieri’s Principle Critical Point Approximation by Differentials Center of Mass Formula Critical Value Arc Length of a Curve Centroid Curly d Area below a Curve Chain Rule Curve Area between Curves Comparison Test Curve Sketching Area of an Ellipse Concave Cusp Area of a Parabolic Segment Concave Down Cylindrical Shell Method Area under a Curve Concave Up Decreasing Function Area Using Parametric Equations Conditional Convergence Definite Integral Area Using Polar Coordinates Constant Term Definite Integral Rules Degenerate Divergent Series Function Operations Del Operator e Fundamental Theorem of Calculus Deleted Neighborhood Ellipsoid GLB Derivative End Behavior Global Maximum Derivative of a Power Series Essential Discontinuity Global Minimum Derivative Rules Explicit Differentiation Golden Spiral Difference Quotient Explicit Function Graphic Methods Differentiable Exponential Decay Greatest Lower Bound Differential -

Approximation of Logarithm, Factorial and Euler- Mascheroni Constant Using Odd Harmonic Series
Mathematical Forum ISSN: 0972-9852 Vol.28(2), 2020 APPROXIMATION OF LOGARITHM, FACTORIAL AND EULER- MASCHERONI CONSTANT USING ODD HARMONIC SERIES Narinder Kumar Wadhawan1and Priyanka Wadhawan2 1Civil Servant,Indian Administrative Service Now Retired, House No.563, Sector 2, Panchkula-134112, India 2Department Of Computer Sciences, Thapar Institute Of Engineering And Technology, Patiala-144704, India, now Program Manager- Space Management (TCS) Walgreen Co. 304 Wilmer Road, Deerfield, Il. 600015 USA, Email :[email protected],[email protected] Received on: 24/09/ 2020 Accepted on: 16/02/ 2021 Abstract We have proved in this paper that natural logarithm of consecutive numbers ratio, x/(x-1) approximatesto 2/(2x - 1) where x is a real number except 1. Using this relation, we, then proved, x approximates to double the sum of odd harmonic series having first and last terms 1/3 and 1/(2x - 1) respectively. Thereafter, not limiting to consecutive numbers ratios, we extended its applicability to all the real numbers. Based on these relations, we, then derived a formula for approximating the value of Factorial x.We could also approximate the value of Euler-Mascheroni constant. In these derivations, we used only and only elementary functions, thus this paper is easily comprehensible to students and scholars alike. Keywords:Numbers, Approximation, Building Blocks, Consecutive Numbers Ratios, NaturalLogarithm, Factorial, Euler Mascheroni Constant, Odd Numbers Harmonic Series. 2010 AMS classification:Number Theory 11J68, 11B65, 11Y60 125 Narinder Kumar Wadhawan and Priyanka Wadhawan 1. Introduction By applying geometric approach, Leonhard Euler, in the year 1748, devised methods of determining natural logarithm of a number [2]. -

Section 7.2, Integration by Parts
Section 7.2, Integration by Parts Homework: 7.2 #1{57 odd 2 So far, we have been able to integrate some products, such as R xex dx. They have been able to be solved by a u-substitution. However, what happens if we can't solve it by a u-substitution? For example, consider R xex dx. For this, we will need a technique called integration by parts. 0 0 From the product rule for derivatives, we know that Dx[u(x)v(x)] = u (x)v(x) + u(x)v (x). Rear- ranging this and integrating, we see that: 0 0 u(x)v (x) = Dx[u(x)v(x)] − u (x)v(x) Z Z Z 0 0 u(x)v (x) dx = Dx[u(x)v(x)] dx − u (x)v(x) dx This gives us the formula needed for integration by parts: Z Z u(x)v0(x) dx = u(x)v(x) − u0(x)v(x) dx; or, we can write it as Z Z u dv = uv − v du In this section, we will practice choosing u and v properly. Examples Perform each of the following integrations: 1. R xex dx Let u = x, and dv = ex dx. Then, du = dx and v = ex. Using our formula, we get that Z Z xex dx = xex − ex dx = xex − ex + C R lnpx 2. x dx Since we do not know how to integrate ln x, let u = ln x and dv = x−1=2. Then du = 1=x and v = 2x1=2, so Z ln x Z p dx = 2x1=2 ln x − 2x−1=2 dx x = 2x1=2 ln x − 4x1=2 + C 3. -

The Logarithmic Chicken Or the Exponential Egg: Which Comes First?
The Logarithmic Chicken or the Exponential Egg: Which Comes First? Marshall Ransom, Senior Lecturer, Department of Mathematical Sciences, Georgia Southern University Dr. Charles Garner, Mathematics Instructor, Department of Mathematics, Rockdale Magnet School Laurel Holmes, 2017 Graduate of Rockdale Magnet School, Current Student at University of Alabama Background: This article arose from conversations between the first two authors. In discussing the functions ln(x) and ex in introductory calculus, one of us made good use of the inverse function properties and the other had a desire to introduce the natural logarithm without the classic definition of same as an integral. It is important to introduce mathematical topics using a minimal number of definitions and postulates/axioms when results can be derived from existing definitions and postulates/axioms. These are two of the ideas motivating the article. Thus motivated, the authors compared manners with which to begin discussion of the natural logarithm and exponential functions in a calculus class. x A related issue is the use of an integral to define a function g in terms of an integral such as g()() x f t dt . c We believe that this is something that students should understand and be exposed to prior to more advanced x x sin(t ) 1 “surprises” such as Si(x ) dt . In particular, the fact that ln(x ) dt is extremely important. But t t 0 1 must that fact be introduced as a definition? Can the natural logarithm function arise in an introductory calculus x 1 course without the