Atlin Northern Mountain Caribou Management and Monitoring Framework: Final Report 2011
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Legacy of a Taku River Tlingit Clan
Gágiwdul.àt: Brought Forth to Reconfirm THE LEGACY OF A TAKU RIVER TLINGIT CLAN Gágiwdul.àt: Brought Forth to Reconfirm THE LEGACY OF A TAKURIVER TLINGIT CLAN Elizabeth Nyman and JeffLeer Yukon Native Language Centre and Alaska Native Language Center 1993 lV © 1993, Yukon Native Language Centre, Alaska Native Language Center, and Elizabeth Nyman Printed in the United States of America All rights reserved Library of Congress Cataloging-in-Publication Data Nyman, Elizabeth, 1915- Gágiwdutàt : The Legacy of a Taku River Tlingit Clan / Elizabeth Nyman and Jeff Leer. p. cm. Includes index. ISBN 1-55500-048-7 1. Tlingit Indians-Legends. 2. Tlingit Indians-Social life and customs. 3. Nyman, Elizabeth, 1915- . 4. Tlingit Indians-Biography. 5. Tlingit language-Texts. 1. Leer, Jeff. Il. Title. E99.T6N94 1993 93-17399 398.2'089972-dc20 CIP First Printing, 1993 1,000 copies Cover photo: Yakadlakw Shà 'Scratched-face Mountain' (no English name) and the Taku River near Atlin, by Wayne Towriss for YNLC Cover design and drawing on title pages by Dixon Jones, UAF IMP ACT Yukon Native Language Centre Alaska Native Language Center Yukon College University of Alaska Fairbanks Box 2799 Fairbanks, Alaska 99775-0120 Whitehorse, Yukon Canada YlA 5K4 The printing of this book was made possible in part by a contribution to the Council for Yukon Indians by the Secretary of State for Canada and Aborigi nal Language Services (Government of Yukon). It is the policy of the University of Alaska to provide equal education and employment opportunities and to provide -

FNESS Strategic Plan
Strategic Plan 2013-2015 At a Glance FNESS evolved from the Society of Native Indian Fire Fighters of BC (SNIFF), which was established in 1986. SNIFF’s initial objectives were to help reduce the number of fire-related deaths on First Nations reserves, but it changed its emphasis to incorporate a greater spectrum of emergency services. In 1994, SNIFF changed its name to First Nations’ Emergency Services Society of BC to reflect the growing diversity of services it provides. Today our organization continues to gain recognition and trust within First Nations communities and within Aboriginal Affairs and Northern Development Canada (AANDC) and other organizations. This is reflected in both the growing demand of service requests from First Nations communities and the development of more government-sponsored programs with FNESS. r e v Ri k e s l A Inset 1 Tagish Lake Teslin 1059 Daylu Dena Atlin Lake 501 Taku River Tlingit r e v Liard Atlin Lake i R River ku 504 Dease River K Fort a e Nelson T r t 594 Ts'kw'aylaxw e c iv h R ik River 686 Bonaparte a se a 687 Skeetchestn e D Fort Nelson R i v e First Nations in 543 Fort Nelson Dease r 685 Ashcroft Lake Dease Lake 592 Xaxli'p British Columbia 593 T'it'q'et 544 Prophet River 591 Cayoose Creek 692 Oregon Jack Creek 682 Tahltan er 683 Iskut a Riv kw r s e M u iv R Finlay F R Scale ra e n iv s i er 610 Kwadacha k e i r t 0 75 150 300 Km S 694 Cook's Ferry Thutade R r Tatlatui Lake i e 609 Tsay Keh Dene v Iskut iv 547 Blueberry River e R Lake r 546 Halfway River 548 Doig River 698 Shackan Location -
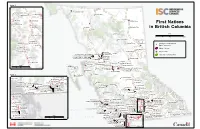
For a Larger Version of the First Nations in British
#! Inset 1 Tagish Lake #! Teslin 502 Liard Atlin Lake #!501 Taku River Tlingit L 594 Ts'kw 'aylaxw iard #! Atlin Lake R 687 Skeetchestn ive #! ! 504 Dease River K r 686 Bonaparte # #! e r t e c iv h R ik #! a se a e D Fort Nelson R ! i # ! 592 Xaxli'p #! 685 Ashcroft v # e 543 Fort Nelson Dease r #! 593 T'it'q'et Lake Dease Lake #! First Nations 591 Cayoose Creek #! 692 Oregon Jack Creek 682 Tahltan #! 544 P rophet River r #! a ive in British Colum bia F R in British Colum bia 683 Iskut r #! kw a r s s e M u e iv r R Finlay R e iv n er i 610 Kw ad acha k Scale i t #! ! S R # 694 Cook's Ferry i v 0 75 150 300 km e r Thutade r e Lake I iv Tatlatui 609 Tsay Keh Dene skut R #! 547 Blueberry River Lake #! 698 Shackan #! #! #! #! 696 Nicom en 546 Halfw ay River 548 Doig River 705 Lytton #! #! Location of First Nation's 699 Nooaitch Main Community #! Williston Fort St John 707 Skuppah #! Lake N Indian Reserve a ! s 542 Saulteau # 706 Siska s #! #! 704 Kanaka Bar #! R Takla i 545 W est Moberly v City or Town e Lake r 532 Kispiox 533 Glen Vow ell 608 Takla 677 Nisga'a Village of New Aiyansh 537 Gitanyow 531 Gitanm aax #! #! Park and Protected Area 679 Nisga'a Village of Gitw inksihlkw #! #!!534 Hagw ilget 678 Nisga'a Village of Laxgalt'sap #!#! # #! 700 Boothroyd ! #! #! 535 Gitsegukla 671 Nisga'a Village of Gingolx#! # ! Babine #! 618 McLeod Lake 536 Gitw ar ngak # e 530 W itset v i Sm ithers 674 Lax Kw 'alaam s R Lake 617 Tl'azt'en ! 701 Boston Bar ! # #! Terrace #!680 Kitselas 728 Yekooche ! # #! # #! 730 Binche W hut'en 673 Metlakatla ena -

P.~Cific· 'R.I..W; ·Pivl.S1po,:
) I' , ,,' , ' f , • ,O~ " by. N~ Seigel. '. C~ HcEwen , " . NORTHERN BIOMES LTD Environme~tal Servic.s Whitehorse', Yukon" for Department of. FiSl.h,El·l",ies, and. ,Oceans ,P.~cific· 'R.i..W; ·pivl.s1po,: , . • r·',',·". , , ~. , . ~ '. ' June... 1,9'84 i ) ACKNOWLEDGEMENTS This project was funded by the Federal Department of Fisheries and Oceans. Fisheries personnel in Whitehorse, Vancouver and Ottawa were most helpful and we would especially like to thank Michael Hunter, Gordon Zealand, Sandy Johnston, Peter Etherton, Elmer Fast, Tim Young, Obert Sweitzer, and Ciunius Boyle. The help and patience of personnel from the Yukon Archives, Hudson's Bay Arohives, and Publio Archives of Canada, partioularly Bob Armstrong, the arohivist in charge of Fisheries documents, is gratefully acknowledged. Interviews with Yukon residents: G.I. Cameron, Charles "Chappie ft Chapman, Silvester Jack, Dorothy Jackson, Elizabeth Nyman, Angela Sidney, George Simmons, Virginia Smarch, Dora Wedge and Ed Whjtehouse provided information that was otherwise not available. Julie Cruikshank suggested useful reference resouroes for Indian fishing information. Aileen Horler and Tim Osler offered suggestions during the embryonic stage of the study. Valuable editorial comments were provided by Gavin Johnston. Sandy Johnston provided ourrent information on the Canada/U.S. Yukon River salmon negotiations. The report was typed by Norma Felker, Sharilyn Gattie and Kelly Wilkinson. ii SUMMARY Prior to the Klondike Gold Rush of 1898, fishing in the Yukon was primarily done by indigenous peoples for subsistenoe. For a number of Indian bands, fish, and partioularly salmon, was the primary food souroe. Contaot with White furtraders initiated a ohange in the Indian lifestyle. -

Resource Atlas for Planning Under the Atlin-Taku Framework Agreement
RESOURCE ATLAS FOR PLANNING UNDER THE ATLIN-TAKU FRAMEWORK AGREEMENT Version 1.5 August, 2009 Resource Atlas Resource Atlas ACKNOWLEDGEMENTS This Atlas was compiled with contributions from many people. Of particular note, maps were produced by Shawn Reed and Darin Welch with the assistance of Dave Amirault, Integrated Land Management Bureau. Descriptive information was mainly based on the report Atlin-Taku Planning Area Background Report: An Overview of Natural, Cultural, and Socio-Economic Features, Land Uses and Resources Management (Horn and Tamblyn 2002), Government of BC websites, and for wildlife the joint Wildlife Habitat Mapping Information Handout May 2009. Atlin-Taku Framework Agreement Implementation Project Page 3 of 87 Resource Atlas Atlin-Taku Framework Agreement Implementation Project Page 4 of 87 TABLE OF CONTENTS Acknowledgements ....................................................................................................................................... 3 Table of Contents ......................................................................................................................................... 5 Introduction ................................................................................................................................................... 7 General Plan Area Description ................................................................................................................... 7 Map 1: Base Information ............................................................................................................................ -

Southern Lakes Fisheries Angler Education and Outreach Campaign - Spring 2020
Southern Lakes Fisheries Angler Education and Outreach Campaign - Spring 2020 Education and Outreach Focussed on Grayling, Northern Pike and Lake Trout at the Lubbock River, Little Atlin Lake and additional accessible locations in the Southern Lakes. Carcross/Tagish Renewable Resources Council June 2020 Campaign and Outreach Facilitated by: Dennis Zimmermann - Big Fish Little Fish Consultants Overview: Increased fishing pressure in the accessible Southern Lakes was already a concern, prior to covid-19. The concern for vulnerable Lubbock River Grayling and Little Atlin Lake Trout was the impetus for this campaign led by the Carcross/Tagish Renewable Resources Council (C/TRRC). The C/TRRC working with partners Carcross/Tagish First Nation (C/TFN) and the Department of Environment (DOE), hired Dennis Zimmermann with Big Fish Little Fish Consultants to develop an outreach and education campaign in the spring of 2020. The outreach and education campaign featured numerous tactics and means by which various messages could be presented to the licenced Yukon angling public. Tactics included the installation of signage at approximately 11 locations, a press release, social media communications and outreach over four weekends between May and June at the Lubbock River and one weekend at Little Atlin Lake. 1 Feedback from anglers and the public has been positive both on social media, in- person responses, and anecdotally comments from the community. Some of the greatest successes of this campaign include: • facilitating a positively focussed and proactive -

Place-Based Salmon Management in Taku River Tlingit Territory
STRIVING TO KEEP A PROMISE: PLACE-BASED SALMON MANAGEMENT IN TAKU RIVER TLINGIT TERRITORY by Susan L. Dain-Owens B.A. Dartmouth College, 2005 A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF ARTS in The Faculty of Graduate Studies (Anthropology) THE UNIVERSITY OF BRITISH COLUMBIA (Vancouver) June, 2008 © Susan L. Dain-Owens, 2008 ABSTRACT The Taku River Tlingit First Nation of Northwest British Columbia harvests salmon for commercial, cultural, and sustenance purposes. In this case study I describe the current co-management process of the Taku River salmon fishery as it exists between the First Nation and the Canadian and Alaskan governments, drawing primarily on ethnographic fieldwork conducted in the summer of 2007. In the past, Tlingit families spent the summer on the lower Taku River and vicinity, fishing as part of the seasonal round. Today many families continue to fish on the Taku, and life downriver is a rhythmic blend of hard work and rest. I experienced the knowledge sharing, cooperation, and flexibility that exists downriver and caught a glimpse of a particular Tlingit worldview. There exists a sense of community on the river between the Tlingit fishers, the non-native fishers, and scientists from both Alaska and Canada. Interaction and cooperation between these stakeholders occurs at different scales from individual to international. In both politics and daily life downriver, worldviews become intertwined in a dynamic play between the groups. Though problems and misunderstandings can arise at these junctures, the potential for knowledge sharing across these boundaries exists and should be recognized. -

Wóoshtin Wudidaa Atlin Taku Land Use Plan
Wóoshtin wudidaa Atlin Taku Land Use Plan Wóoshtin wudidaa Atlin Taku Land Use Plan July 19, 2011 Contact information: For more information on the Atlin Taku Land Use Plan, please contact: Taku River Tlingit First Nation Province of British Columbia Land and Resources Department 3726 Alfred Ave Box 132 Smithers, BC Atlin, BC V0J 2N0 V0W 1A0 250-651-7900 250-847-7260 www.trtfn.yikesite.com www.ilmb.gov.bc.ca/slrp/lrmp/smithers/atlin_ta ku/index.html Acknowledgements The Atlin Taku Land Use Plan reflects the vision, hard work and dedication of many individuals and groups. Special recognition is given to individuals on the working groups representing the Province of BC and the Taku River Tlingit First Nation: . The Joint Land Forum –the bilateral government-to-government body responsible for developing the Land Use Plan, included the following members: Sue Carlick (TRTFN co- chair), Bryan Jack, John Ward and Melvin Jack representing the TRTFN; and Kevin Kriese (BC co-chair), Brandin Schultz (MOE), Loren Kelly (MEMPR, Alternate), Åsa Berg (Atlin Community Representative), and Rose Anne Anttila (Atlin Community Representative, Alternate) representing the Province of BC. Representatives of the Atlin Taku Technical Working Group acting for the TRTFN were Bryan Evans (TRTFN Team Leader), Julian Griggs, Kim Heinemeyer, Nicole Gordon and Jerry Jack. Representatives of the Technical Working Group acting for BC were James Cuell (BC Team Leader), Fred Oliemans, Lisa Ambus, Katie von Gaza and Tony Pesklevits. The Responsible Officials under the Framework Agreement included Gary Townsend, Assistant Deputy Minister, Integrated Land Management Bureau; and John Ward, Spokesperson, Taku River Tlingit First Nation. -

Taku River Tlingit First Nation V Canada (Attorney General)
SUPREME COURT OF YUKON Citation: Taku River Tlingit First Nation v Date: 20160128 Canada (Attorney General) S.C. No. 13-A0159 2016 YKSC 7 Registry: Whitehorse Between: TAKU RIVER TLINGIT FIRST NATION Plaintiff And ATTORNEY GENERAL OF CANADA Defendant Before Mr. Justice R.S. Veale Appearances: Stephen Walsh Counsel for the plaintiff Suzanne Duncan and Jonathan Gorton Counsel for the defendant REASONS FOR JUDGMENT INTRODUCTION [1] In this summary trial, the Taku River Tlingit First Nation (“Taku River Tlingit”) applies for two declarations against the Attorney General of Canada (“Canada”): the first is a declaration that Canada, having officially accepted for negotiation the Taku River Tlingit comprehensive land claim, which includes traditional territory in Yukon, is required, acting honourably, to participate in processes of negotiation towards a just settlement of the transboundary claim in Yukon. The second is a declaration that the honour of the Crown requires Canada to take steps within its power to protect and Taku River Tlingit First Nation v Canada (Attorney General) Page 2 2016 YKSC 7 preserve Taku River Tlingit rights and interests in and to its claimed territory in Yukon, pending settlement. [2] Canada opposes the application on several grounds. Firstly, that Canada is negotiating with the Taku River Tlingit in British Columbia and the honour of the Crown is met; secondly, that the honour of the Crown does not give rise to a duty to negotiate; thirdly, that the Crown prerogative permits Canada to decide when and how to negotiate; and fourthly, that ss. 49 and 50 of the Yukon Act do not apply to this case. -

Robert C. (Bob) Harris
Robert C. (Bob) Harris An Inventory of Material In the Special Collections Division University of British Columbia Library © Special Collections Division, University Of British Columbia Library Vancouver, BC Compiled by Melanie Hardbattle and John Horodyski, 2000 Updated by Sharon Walz, 2002 R.C. (Bob) Harris fonds NOTE: Cartographic materials: PDF pages 3 to 134, 181 to 186 Other archival materials: PDF pages 135 to 180 Folder/item numbers for cartographic materials referred to in finding aid are different from box/file numbers for archival materials in the second half of the finding aid. Please be sure to note down the correct folder/item number or box/file number when requesting materials. R. C. (Bob) Harris Map Collection Table of Contents Series 1 Old Maps – Central B. C. 5-10 Series 2 Old Maps – Eastern B. C. 10-17 Series 3 Old Maps – Miscellaneous 17-28 Series 4 Central British Columbia maps 28-39 Series 5 South-central British Columbia maps 39-50 Series 6 Okanagan maps 50-58 Series 7 Southern Interior maps 58-66 Series 8 Old Cariboo maps [i.e. Kootenay District] 66-75 Series 9 Additional Cariboo maps 75-77 Series 10 Cariboo Wagon Road maps 77-90 Series 11 Indian Reserve maps 90-99 Series 12 North-eastern British Columbia maps [i.e. North-western] 99-106 Series 13 BC Northern Interior maps 106-116 Series 14 West Central British Columbia maps 116-127 Series 15 Bella Coola and Chilcotin maps 127-130 Series 16 Series 16 - Lillooet maps 130-133 -2 - - Robert C. (Bob) Harris - Maps R.C. -

Atlin Past and Present
Photo courtesy of Edith Sidler Edith of courtesy Photo Bergeron JF / Tourism BC Northern of courtesy Photo Columbia British Atlin, www.sitesandtrailsBC.ca Ph: 250 638-5100 250 Ph: [email protected] Email: Branch Trails and Sites Recreation For More Information More For www.environmentyukon.gov.yk.ca 5648 ex. 1-800-661-0408 Yukon): (in Free Toll 867-667-5648 Ph: Yukon Parks Yukon Email: [email protected] Email: 651-7522 250 Ph/Fax: Atlin Historical Society Historical Atlin Email: [email protected] Email: 651-7522 250 Ph/Fax: Atlin Visitor Association Visitor Atlin www.env.gov.bc.ca/bcparks 1-800-689-9025 Free: Toll Visitors Map and Guide and Map Visitors BC Parks BC campfire. Be aware of the Fire Danger Rating before lighting a a lighting before Rating Danger Fire the of aware Be 1-888-798-FIRE (3473) 1-888-798-FIRE call Yukon the In Past and Present and Past province-wide, forest fire emergency phone number. phone emergency fire forest province-wide, . This is a free, free, a is This . 1-800-663-5555 call Columbia British In Atlin Should you spot a forest fire: forest a spot you Should Forest Fires Forest Stay Safe In Bear Country Mining Properties Bears feel threatened if surprised hike in a group and The Atlin area has a number of mining properties, many make loud noises. Whistle, talk, sing, or carry noise of which are on private property and may be dangerous. makers such as bells or a can containing stones. In dense Obey all posted signs on mining properties. -

CTFN Chapter 13
CHAPTER 13 - HERITAGE 13.1.0 Objectives 13.1.1 The objectives of this chapter are as follows: 13.1.1.1 to promote public awareness, appreciation and understanding of all aspects of culture and heritage in the Yukon and, in particular, to respect and foster the culture and heritage of Yukon Indian People; 13.1.1.2 to promote the recording and preservation of traditional languages, beliefs, oral histories including legends, and cultural knowledge of Yukon Indian People for the benefit of future generations; 13.1.1.3 to involve equitably Yukon First Nations and Government, in the manner set out in this chapter, in the management of the Heritage Resources of the Yukon, consistent with a respect for Yukon Indian values and culture; 13.1.1.4 to promote the use of generally accepted standards of Heritage Resources management, in order to ensure the protection and conservation of Heritage Resources; 13.1.1.5 to manage Heritage Resources owned by, or in the custody of, Yukon First Nations and related to the culture and history of Yukon Indian People in a manner consistent with the values of Yukon Indian People, and, where appropriate, to adopt the standards of international, national and territorial Heritage Resources collections and programs; 13.1.1.6 to manage Heritage Resources owned by, or in the custody of, Government and related to the culture and history of Yukon Indian People, with respect for Yukon Indian values and culture and the maintenance of the integrity of national and territorial Heritage Resources collections and programs; 13.1.1.7