A General Approach to Cis-Fused Sesquiterpene
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

At the University of Edinburgh
This thesis has been submitted in fulfilment of the requirements for a postgraduate degree (e.g. PhD, MPhil, DClinPsychol) at the University of Edinburgh. Please note the following terms and conditions of use: This work is protected by copyright and other intellectual property rights, which are retained by the thesis author, unless otherwise stated. A copy can be downloaded for personal non-commercial research or study, without prior permission or charge. This thesis cannot be reproduced or quoted extensively from without first obtaining permission in writing from the author. The content must not be changed in any way or sold commercially in any format or medium without the formal permission of the author. When referring to this work, full bibliographic details including the author, title, awarding institution and date of the thesis must be given. Functionalisable Cyclopolymers by Ring-Closing Metathesis Mohammed Alkattan BSc, MSc Drug Chemistry A thesis submitted at the University of Edinburgh and the University of Glasgow for the Degree of Doctor of Philosophy 2019 Abstract Post‐polymerisation modification of polymers is extremely beneficial in terms of designing brand new synthetic pathways toward functional complex polymers. While many chemical groups could provide a platform for chemical functionalisation, arguably one of the most versatile groups is the olefin functionality. This could be significant as the olefins do not readily interfere with common polymerisation techniques such as ring-opening polymerisation (ROP) but can be transformed into a broad range of functional groups. Ring-Closing Metathesis (RCM) is a powerful method for the preparation of cyclic compounds by the formation of new carbon- carbon double bonds. -

Recent Advances on Mechanistic Studies on C–H Activation
Open Chem., 2018; 16: 1001–1058 Review Article Open Access Daniel Gallego*, Edwin A. Baquero Recent Advances on Mechanistic Studies on C–H Activation Catalyzed by Base Metals https:// doi.org/10.1515/chem-2018-0102 received March 26, 2018; accepted June 3, 2018. 1Introduction Abstract: During the last ten years, base metals have Application in organic synthesis of transition metal- become very attractive to the organometallic and catalytic catalyzed cross coupling reactions has been positioned community on activation of C-H bonds for their catalytic as one of the most important breakthroughs during the functionalization. In contrast to the statement that new millennia. The seminal works based on Pd–catalysts base metals differ on their mode of action most of the in the 70’s by Heck, Noyori and Suzuki set a new frontier manuscripts mistakenly rely on well-studied mechanisms between homogeneous catalysis and synthetic organic for precious metals while proposing plausible chemistry [1-5]. Late transition metals, mostly the precious mechanisms. Consequently, few literature examples metals, stand as the most versatile catalytic systems for a are found where a thorough mechanistic investigation variety of functionalization reactions demonstrating their have been conducted with strong support either by robustness in several applications in organic synthesis [6- theoretical calculations or experimentation. Therefore, 12]. Owing to the common interest in the catalysts mode we consider of highly scientific interest reviewing the of action by many research groups, nowadays we have a last advances on mechanistic studies on Fe, Co and Mn wide understanding of the mechanistic aspects of precious on C-H functionalization in order to get a deep insight on metal-catalyzed reactions. -

2.1 the 18-ELECTRON RULE the 18E Rule1 Is a Way to Help Us Decide
30 GENERAL PROPERTIES OF ORGANOMETALLIC COMPLEXES 2.1 THE 18-ELECTRON RULE The 18e rule1 is a way to help us decide whether a given d-block transition metal organometallic complex is likely to be stable. Not all the organic formulas we can write down correspond to stable species. For example, CH5 requires a 5-valent carbon and is therefore not stable. Stable compounds, such as CH4,have the noble gas octet, and so carbon can be thought of as following an 8e rule. This corresponds to carbon using its s and three p orbitals to form four filled bonding orbitals and four unfilled antibonding orbitals. On the covalent model, we can consider that of the eight electrons required to fill the bonding orbitals, four come from carbon and one each comes from the four H substituents. We can therefore think of each H atom as being a 1e ligand to carbon. To assign a formal oxidation state to carbon in an organic molecule, we impose an ionic model by artificially dissecting it into ions. Each electron pair in any bond is assigned to the most electronegative of the two atoms or groups that constitute the bond. For methane, this dissection gives C4− + 4H+, with carbon as the more electronegative element. This makes methane an 8e compound with an oxidation state of −4, usually written C(-IV). Note that the net electron count always remains the same, whether we adopt the covalent (4e {Catom}+4 × 1e {4H atoms}=8e) or ionic (8e{C4−ion}+4 × 0e{4H+ions}=8e) model. -

Assessing the Feasibility of a One-Pot, Tandem Olefin Metathesis and Isomerization Sequence to Synthesize Conjugated Aromatic Olefins
University of Tennessee at Chattanooga UTC Scholar Student Research, Creative Works, and Honors Theses Publications 8-2016 Assessing the feasibility of a one-pot, tandem olefin metathesis and isomerization sequence to synthesize conjugated aromatic olefins Ajay D. Makwana University of Tennessee at Chattanooga, [email protected] Follow this and additional works at: https://scholar.utc.edu/honors-theses Part of the Chemistry Commons Recommended Citation Makwana, Ajay D., "Assessing the feasibility of a one-pot, tandem olefin metathesis and isomerization sequence to synthesize conjugated aromatic olefins" (2016). Honors Theses. This Theses is brought to you for free and open access by the Student Research, Creative Works, and Publications at UTC Scholar. It has been accepted for inclusion in Honors Theses by an authorized administrator of UTC Scholar. For more information, please contact [email protected]. Assessing the Feasibility of a One-Pot, Tandem Olefin Metathesis and Isomerization Sequence to Synthesize Conjugated Aromatic Olefins Ajay D. Makwana Departmental Honors Thesis The University of Tennessee at Chattanooga Department of Chemistry Project Director: Dr. Kyle S. Knight Examination Date: April 4, 2016 Members of Examination Committee: Dr. John P. Lee Dr. Han J. Park Dr. David Giles 1 Abstract The synthesis of substituted phenylpropene dimers using a one-pot, tandem olefin metathesis and isomerization sequence has been studied. This sequence relies on the facilitated, in-situ conversion of a ruthenium carbene species (Ru=C) to a ruthenium hydride species (Ru-H) upon addition of an inorganic hydride source. Three separate reactions occur within one reaction flask: 1) olefin metathesis of the starting phenylpropene to yield phenylpropene dimer via Ru=C catalyst, 2) conversion of Ru=C to Ru-H via addition of an inorganic hydride source, 3) isomerization of phenylpropene dimer via insertion and β-hydride elimination to yield conjugated product. -

Wilkinson's Catalyst
Homogeneous Catalytic Processes Hydroformylation RCH2CH2 O RCH CH2 + CO + H2 Co(I), Rh(I) or Pt(II) H Oxidation H3C O H2CCH2 + O2 Pd(II) or Cu(II) H Carbonylation H3C O - CH3OH + CO [RhI2(CO)2] OH Hydrocyanation [Ni{P(OR) } ] H CCC CH + 2HCN 3 4 NCCH CH CH CH CN 2 H H 2 2 2 2 2 Cyclotrimerization Ni(acac) 3 HC CH 2 Hydrogenation of Alkenes The most commonly used catalyst is the Wilkinson’s Catalyst Many alkenes are hydrogenated with hydrogen at 1atm pressure or less . Wilkinson’s catalyst is highly sensitive to the nature of the phosphine ligand and the alkene substrate. Analogous catalysts with alkyl phosphine ligands are inactive Highly hindered alkenes and ethylene are not hydrogenated by the catalyst Wilkinson’s Catalyst Ph3P PPh3 R CH3 Rh H2 Ph3P Cl Reductive Elimination Oxidative Addition R H H PPh3 H PPh3 Rh Rh Ph3P Cl Ph3P Cl PPh3 PPh3 Ligand Dissociation Ligand Association PPh3 PPh3 H R H PPh3 Rh H PPh3 Cl Rh Ph3P Cl PPh3 H Migratory Insertion H PPh3 Rh R Ph3P Cl Alkene Coordination R Hydroformylation Catalyst + CO + H2 RCH2CH2CHO R A less common,,pppyy but more appropriate name is hydrocarbonylation Both cobalt and rhodium complexes are used as catalysts. Alkene iitiisomerization, alkene hdhydrogena tion and ftiformation of bhdbranched alde hy des are the possible side reactions. Cobalt catalysts operate at 150 ºC and 250 atm, whereas Rhodium catalysts operate at moderate temperatures and 1 atm. Rhodium catalysts promotes the formation of linear aldehydes. Cobalt catalysts do so if modified with alkylphosphine ligands. -

UC Berkeley UC Berkeley Electronic Theses and Dissertations
UC Berkeley UC Berkeley Electronic Theses and Dissertations Title Synthetic Strategies toward Aconitine-type and Hetisine-type Diterpenoid Alkaloids Permalink https://escholarship.org/uc/item/2ws9p3b7 Author Pflueger, Jason Jon Publication Date 2016 Peer reviewed|Thesis/dissertation eScholarship.org Powered by the California Digital Library University of California Synthetic Strategies toward Aconitine-type and Hetisine-type Diterpenoid Alkaloids By Jason Jon Pflueger A dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in Chemistry in the Graduate Division of the University of California, Berkeley Committee in Charge: Professor Richmond Sarpong, Chair Professor Thomas Maimone Professor Leonard Bjeldanes Fall 2016 Abstract Synthetic Strategies toward Aconitine-type and Hetisine-type Diterpenoid Alkaloids by Jason Jon Pflueger Doctor of Philosophy in Chemistry University of California, Berkeley Professor Richmond Sarpong, Chair Diterpenoid alkaloid natural products, isolated from plants in the Aconitum, Delphinium, Consolida, and Spiraea genera, possess complex, caged, highly oxygenated skeletons and display potent biological activities through interactions with voltage-gated ion channels. Several of these alkaloids are currently used clinically for the treatment of arrhythmia, while others act as incredibly potent neurotoxins. Until recently, there were very few successful total syntheses of any diterpenoid alkaloid natural products, a testament to the structural complexity of these -

Literature Seminar#1
Literature Seminar (B4 part) January 17, 2012 Seiko KITAHARA (B4) Total synthesis of Laulimalide OH H O O 15 23 20 17 O O OH 1 H H O 3 5 9 1: Laulimalide (fijianolide B) Contents 1. Introduction 2. Previous Total Synthesis 2-1. Retrosynthetic Analysis 3. Trost's Total Synthesis -toward the atom economy- 3-1. What is "synthetic efficiency" ?? 3-2. Retrosynthetic Analysis 3-3. Total Synthesis Marine Sponge, 3-4. Asymmetric Direct Aldol Reaction Cacospongia mycofijiensis via a Dinuclear Zn Catalyst 3-5. Rh-Catalyzed Cycloisomerization 3-6. Ru-Catalyzed Alkene-Alkyne Coupling Barry M. Trost 1. Introduction OH OH H O H O 15 O OH 20 O 23 17 17 15 20 O O OH 1 H H On exposure to acid O O O 5 9 3 (within two hours) H H O 1: Laulimalide : intrinsically unstable isolaulimalide (fijianolide B) (fijianolide A) <Isolation> -From various marine sponges such as hyattela sp., Cacospongia mycofijiensis, f asciospongia rimosa a marine sponge in the genus Dactylospongia a nudibranch, Chromodoris lochi with its tetrahydrofuran containing isomer isolaulimalide <Structure> -Determined NMR analysis and X-ray crystallographic analysis Corley, D. G et al. J. Org. Chem. 1988, 53, 3644. Quinoa, E et al. J. Org. Chem. 1988, 53, 3642. -20-membered macrolide <Biological activity> -like Taxol (paclitaxel), induces microtubule polymerization and stabilization -unlike Taxol (paclitaxel), retains activity in multidrug resistant cell lines -binds to a different site than other known microtubule stabilzers suggesting new opportunities forchemotherapy ?? <Total synthesis> -Hot topic for over a decade (more than 10 reports !) due to its significant clinical potential its strict natural supply unique and complex molecular architecture Ghosh, A. -

I the Tandem Chain Extension-Acylation Reaction II Synthesis of Papyracillic Acid A
University of New Hampshire University of New Hampshire Scholars' Repository Doctoral Dissertations Student Scholarship Fall 2013 I The tandem chain extension-acylation reaction II Synthesis of papyracillic acid A: Application of the tandem homologation- acylation reaction III Synthesis of tetrahydrofuran-based peptidomimetics Carley Meredith Spencer Follow this and additional works at: https://scholars.unh.edu/dissertation Recommended Citation Spencer, Carley Meredith, "I The tandem chain extension-acylation reaction II Synthesis of papyracillic acid A: Application of the tandem homologation-acylation reaction III Synthesis of tetrahydrofuran-based peptidomimetics" (2013). Doctoral Dissertations. 749. https://scholars.unh.edu/dissertation/749 This Dissertation is brought to you for free and open access by the Student Scholarship at University of New Hampshire Scholars' Repository. It has been accepted for inclusion in Doctoral Dissertations by an authorized administrator of University of New Hampshire Scholars' Repository. For more information, please contact [email protected]. I. THE TANDEM CHAIN EXTEN SION - AC YL ATION REACTION II. SYNTHESIS OF PAPYRACILLIC ACID A: APPLICATION OF THE TANDEM HOMOLOGATION-ACYLATION REACTION III. SYNTHESIS OF TETRAHYDROFURAN-BASED PEPTIDOMIMETICS BY Carley Meredith Spencer B.A., Connecticut College, 2008 DISSERTATION Submitted to the University of New Hampshire in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy in Chemistry September 2013 UMI Number: 3575989 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a complete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. -
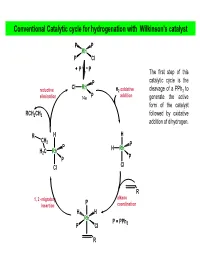
Conventional Catalytic Cycle for Hydrogenation with Wilkinson's Catalyst
Conventional Catalytic cycle for hydrogenation with Wilkinson’s catalyst P P Rh P Cl PP The first step of this P catalytic cycle is the Cl Rh reductive H2 oxidative cleavage of a PPh3 to elimination 14e P addition generate the active form of the catalyst RCH2CH3 followed by oxidative addition of dihydrogen. R H H CH 2 P P H Rh H2C Rh P P Cl Cl R alkene 1, 2 -migratory P insertion coordination H H Rh P = PPh P Cl 3 R AJELIAS L7-S18 catalytic cycle for hydrogenation H H2 oxidative P P addition H P Kinetic studies have Rh Rh shown that the P Cl P Cl dissociation of PPh3 P from the distorted P square planar complex PP (due to trans RhCl(PPh3)3 in effect of H ) H benzene occurs only to a very small extent H2 oxidative P (k = 2.3 × 10–7 M at P addition HRh Cl Rh 25°C), and P P under an atmosphere Cl of H2, a solution of RCH2CH3 RhCl(PPh3)3 becomes reductive alkene yellow as a result of elimination the oxidative addition of H2 to give cis- R H P CH2 H2RhCl(PPh3)3. P H H H2CRh Rh P 1, 2 -migratory P Cl insertion Cl R The trans effect is the labilization (making unstable) of ligands that are trans to certain other ligands, which can thus be regarded as trans-directing ligands. The intensity of the trans effect (as measured by the increase in rate of substitution of the trans ligand) follows this sequence: H2O, OH− < NH3 < py < Cl− < Br− < I−, < PR3, CH3− < H−, NO, CO AJELIAS L7-S19 Relative reactivity of alkenes for homogenous catalytic hydrogenation • Cis alkenes undergo hydrogenation more readily than trans alkenes •Internal and branched alkenes -

Basics of Catalysis and Kinetics
Basics of Catalysis and Kinetics Nobel laureates in catalysis: Haber (1918) Ziegler and Natta (1963) Wilkinson, Fischer (1973) Knowles, Noyori, Sharpless (2001) Grubbs, Schrock, Chauvin (2006) Ertl (2007) Heck, Negishi, Suzuki (2010) G. Poli Definition The concept of catalyst was first introduced by Berzelius in the 19th century. Subsequently, Ostwald came up with the definition that we still use today: “A catalyst is a substance that increases the rate of a chemical reaction, without being consumed or produced”… Berzelius, J. J. Ann. Chim. Phys. 1836, 61, 146-151 Ostwald, W. Z. Phys. Chem. 1894, 15, 705-706 G. Poli The Catalyst and the Rate of a Reaction ΔG non catalyzed catalyzed A B Reaction coordinate The catalyst changes only the rate of the reaction, and does not change the thermodynamics. However, the temperature can affect equilibrium concentrations: ΔG = ΔH - T ΔS G. Poli Historical basis for the Analysis of Catalytic Kinetics The Michaelis-Menten Kinetics G. Poli Thermal, Catalyzed, Inhibited, and Promoted Reactions I II III IV uncatalyzed catalysis inhibition stoichimetric process (no catalysis) promotion (no catalysis) A A A A Acat Bcat Ainhib Binhib Aprom B B (+ cat) B Bprom SM is stabilized SM is stabilized more than TS --> a further reaction is less than TS the uncatalyzed path needed to liberate B is preferred G. Poli Thermal, Catalyzed, Inhibited, and Promoted Reactions O NH HN CCl3 Ar O CCl3 xylenes 140°C type I Ar CCl3 CCl3 O NH PdCl2(PhCN)2 (5 mol%) O NH type II THF, rt N H H N B N N N N B Pt N N 1. -

Decomposition of Ruthenium Olefin Metathesis Catalyst
catalysts Review Decomposition of Ruthenium OlefinOlefin Metathesis CatalystMetathesis Catalyst Magdalena Jawiczuk 1,, Anna Anna Marczyk Marczyk 1,21,2 andand Bartosz Bartosz Trzaskowski Trzaskowski 1,* 1,* 1 1 CentreCentre of of New New Technologies, Technologies, University University of of Warsaw, Warsaw, Banacha Banacha 2c, 2c, 02-097 02-097 Warsaw, Warsaw, Poland; [email protected]@cent.uw.edu.pl (M.J.); (M.J.); [email protected] [email protected] (A.M.) (A.M.) 2 Faculty of Chemistry, University of Warsaw, Pasteura 1, 02-093 Warsaw, Poland 2 Faculty of Chemistry, University of Warsaw, Pasteura 1, 02-093 Warsaw, Poland * Correspondence: [email protected] * Correspondence: [email protected] Received: 28 28 June 2020; Accepted: 02 2 AugustAugust 2020;2020; Published:Published: 5date August 2020 Abstract: RutheniumRuthenium olefin olefin metathesis metathesis catalysts catalysts are are one one of of the most commonly used class of catalysts. There There are are multiple multiple reviews reviews on on their their us useses in in various branches of chemistry and other sciences but a detailed review of their decomposition is missing, despite a large number of recent and important advances advances in in this this field. field. In In particular, particular, in in the the last last five five years years several several new new mechanism mechanism of decomposition,of decomposition, both both olefin-driven olefin-driven as well as well as induc as induceded by external by external agents, agents, have have been been suggested suggested and usedand usedto explain to explain differences differences in the decomposition in the decomposition rates and rates the metathesis and the metathesis activities activitiesof both standard, of both N-heterocyclicstandard, N-heterocyclic carbene-based carbene-based systems and systems the recently and the developed recently developed cyclic alkyl cyclic amino alkyl carbene- amino containingcarbene-containing complexes. -

Chapter 1 the Basic Chemistry of Organopalladium Compounds
Chapter 1 The Basic Chemistry of Organopalladium Compounds 1.1 Characteristic Features of Pd-Promoted or -Catalyzed Reactions There are several features which make reactions involving Pd catalysts and reagents particularly useful and versatile among many transition metals used for organic synthesis. Most importantly, Pd catalysts offer an abundance of possibilities of carbon–carbon bond formation. Importance of the carbon–carbon bond forma- tion in organic synthesis needs no explanation. No other transition metals can offer such versatile methods of the carbon–carbon bond formations as Pd. Tol- erance of Pd catalysts and reagents to many functional groups such as carbonyl and hydroxy groups is the second important feature. Pd-catalyzed reactions can be carried out without protection of these functional groups. Although reactions involving Pd should be carried out carefully, Pd reagents and catalysts are not very sensitive to oxygen and moisture, and even to acid in many reactions catalyzed by Pd–phosphine complexes. It is enough to apply precautions to avoid oxidation of coordinated phosphines, and this can be done easily. On the other hand, the Ni(0) complex is extremely sensitive to oxygen. Of course, Pd is a noble metal and expensive. Its price frequently fluctuates drastically. A few years ago, Pd was more expensive than Pt and Au but cheaper than Rh. As of October 2003, the comparative prices of the noble metals were: Pd (1), Au (1.8), Rh (2.8), Pt (3.3), Ru (0.2). Recently the price of Pd has dropped dramatically, and Pt is currently the most expensive noble metal. Also, the toxicity of Pd has posed no serious problem so far.