Lammerite, Cur(Asoo)R, a Modulated Close-Packed Structure
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Download the Scanned
NOTICES 219 NICKEL, AN HISTORICAL REVUE. F. B. Howeno-Wnrru. D. Van Nostrand Co., Inc., 120 Alexander St., Princeton, N. J. 350 pp. 5 Appendixes. 1963. 9695. This is a literary toltr ile force, broadly conceived, exhaustively executed and elegantly illuminated. The author, who has spent his life in the world of nickel, was at his retire- ment a director of the International Nickel Company (Mond) Ltd. In this monograph he has attempted to include (and I suspect iargely successfully) everything that is known on the discovery and development of nickel as a commercial metal, adding, en route, large dollops of data on such relevant topics as the earth's interior, composition of early bronze alloys, meteorites, early mining, extractive metallurgy, development of armor and ord- nance, electroplating, international business operations, coinage, aircraft engines, world airplane speed records, properties of steels, atomic structure and medieval altarpieces. These various reflections are combined into a finely faceted whole, highlighted by numer- ous germane quotations from original sources. Illustrations are numerous and first class, including several in living color and a number of fold-out charts. The whole is a sumptious production. ewh DER MINERALIENSAMMLER. WBnNnn LrneBn. 225 pages, 56 figures, 28 plates (incl 8 in color), rear pocket with 5 maps and 9 "exploded" crystal drawings for cut- out reassembling. OH-Verlag, Liinggasse 57, Thun, Switzerland. $6.45. 1963. This is a complete guide and introduction to mineralogy for the beginning collector. Essentially it is in two parts: Chapters I-IX deal with elementary basic principles of miner- alogy (formation of minerals, chemical composition, crystals, properties, mineral determi- nation) and with mineral collecting (equipment, cleaning, preservation and display, pur- chase and swapping). -

Mineral Processing
Mineral Processing Foundations of theory and practice of minerallurgy 1st English edition JAN DRZYMALA, C. Eng., Ph.D., D.Sc. Member of the Polish Mineral Processing Society Wroclaw University of Technology 2007 Translation: J. Drzymala, A. Swatek Reviewer: A. Luszczkiewicz Published as supplied by the author ©Copyright by Jan Drzymala, Wroclaw 2007 Computer typesetting: Danuta Szyszka Cover design: Danuta Szyszka Cover photo: Sebastian Bożek Oficyna Wydawnicza Politechniki Wrocławskiej Wybrzeze Wyspianskiego 27 50-370 Wroclaw Any part of this publication can be used in any form by any means provided that the usage is acknowledged by the citation: Drzymala, J., Mineral Processing, Foundations of theory and practice of minerallurgy, Oficyna Wydawnicza PWr., 2007, www.ig.pwr.wroc.pl/minproc ISBN 978-83-7493-362-9 Contents Introduction ....................................................................................................................9 Part I Introduction to mineral processing .....................................................................13 1. From the Big Bang to mineral processing................................................................14 1.1. The formation of matter ...................................................................................14 1.2. Elementary particles.........................................................................................16 1.3. Molecules .........................................................................................................18 1.4. Solids................................................................................................................19 -
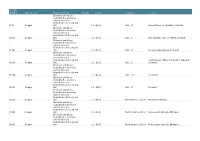
BRSUG Number Mineral Name Hey Index Group Hey No
BRSUG Number Mineral name Hey Index Group Hey No. Chem. Country Locality Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-37 Copper Au) 1.1 4[Cu] U.K., 17 Basset Mines, nr. Redruth, Cornwall Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-151 Copper Au) 1.1 4[Cu] U.K., 17 Phoenix mine, Cheese Wring, Cornwall Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-280 Copper Au) 1.1 4[Cu] U.K., 17 County Bridge Quarry, Cornwall Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and South Caradon Mine, 4 miles N of Liskeard, B-319 Copper Au) 1.1 4[Cu] U.K., 17 Cornwall Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-394 Copper Au) 1.1 4[Cu] U.K., 17 ? Cornwall? Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-395 Copper Au) 1.1 4[Cu] U.K., 17 Cornwall Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-539 Copper Au) 1.1 4[Cu] North America, U.S.A Houghton, Michigan Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-540 Copper Au) 1.1 4[Cu] North America, U.S.A Keweenaw Peninsula, Michigan, Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-541 Copper Au) 1.1 4[Cu] North America, U.S.A Keweenaw Peninsula, Michigan, Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, -

Alteration - Porphyries
ALTERATION - PORPHYRIES Occurs with minor mineralization in the deeper Potassic parts of some porphyry systems, and is a host to mineralization in porphyry deposits associated with alkaline intrusions © Copyright Spectral International Inc. Sodic, sodic-calcic Propylitic Potassic alteration shows Actinolite, biotite, phlogopite, epidote, iron- chlorite, Mg-chlorite, muscovite, quartz, anhydrite, magnetite. Albite, actinolite, diopside, quartz, Fe-chlorite, Mg-chlorite, epidote, actinolite, magnetite, titanite, chlorite, epidote, calcite, illite, montmorillonite, albite, pyrite. scapolite Intermediate argillic alteration generally This is an intense alteration phase, often in the Copyright Spectral International Inc.© forms a structurally controlled to upper part of porphyry systems. It can also form envelopes around pyrite-rich veins that Phyllic alteration commonly forms a widespread overprint on other types of cross cut other alteration types. peripheral halo around the core of alteration in many porphyry systems porphyry deposits. It may overprint earlier potassic alteration and may host substantial mineralization ADVANCED ARGILLIC Phyllic INTERMEDIATE ARGILLIC The minerals associated with this type The detectable minerals include are higher temperature and include illite, muscovite, dickite, kaolinite, pyrophyllite, quartz, andalusite, Fe-chlorite, Mg-chlorite, epidote, diaspore, corundum, alunite, topaz, montmorillonite, calcite, and pyrite tourmaline, dumortierite, pyrite, and hematite EXOTIC COPPER www.e-sga.org/ CU PHOSPHATES Cu Cu CARBONATES CHLORIDES Azurite, malachite, aurichalcite, rosasite Atacamite, connellite, and cumengite © Copyright Spectral International Inc. Cu-ARSENATES EXOTIC COPPER Cu-SILICATES Cu-SULFATES bayldonite, antlerite, chenevixite chrysocolla dioptase brochantite, conichalcite chalcanthite clinoclase papagoite shattuckite cyanotrichite olivenite kroehnite, spangolite © Copyright Spectral International Inc. © Copyright Spectral International Inc. LEACH CAP MINERALS . -

Papagoite, a New Copper-Bearing Mineral from Ajo, Artzona*
THE AMERICAN MINERA]-OGIST, VOL. 45, MAY-JUNE, 1960 PAPAGOITE, A NEW COPPER-BEARING MINERAL FROM AJO, ARTZONA* C. OssonNn llutrow, StanJord'(Iniaersi'ty, Stanford', California, .qNpANcBr-rN.q, C. Vr-rsrors, U. S. Geologi'colSuroey, Washington,D. C. AssrRAcr thereof. to (100) was noted in thin sections but it was not evident in crystals or fragments Papagoiteismonoclinicprismatic,spacegroup Cm,C2,otC2fm,witha:12'9lA'b:ll'48' pattern is re- ,:+.05, all values +0.01 A; g:100'38'; aibic:1.124;l:0.408. A powder of the five corded and it has been indexed up to 2A-65o; the d-spacings and intensities (8)' strongest lines are: 2.874 i\ G0), 4.29 (9),2.204 (9),2.795 (8), and 3 '44 The mineral has a cerulean biue color in hand specimen and is distinctly pleochroic. : -a : 2Y : 78" dispersion faint' Biaxial, negative; a : 1.607, B : l'641, t l'67 2, 7 0'065; ; r)v;X:very pale glaucousgreen to colorless,Y: cendreblue, Z:Venice gteen;ZlYlK; to the Xnr:++" both in the acute angle,and Z:b'Y is very nearly normal "iXnd:35', 4 { CaCuAl[SiOr],(OH) ' I . in which The mineral has been named for the Indian tribe that once inhabited the region the mining center of Ajo, Arizona, is situated' Occunnoncr The papagoite-bearingspecimens were found at Ajo, Arizona, in an isolatedpocket 150ft. below ground-levelat the 1750level in an areathat has now been entirely removed. The nearest high-grade sulfide ore now lies 50 ft. below and 1000ft. -

NEW MINERAL NAMES Schoderite, Metaschoderite D. M. H,Q,Usnn
NEW MINERAL NAMES Schoderite, Metaschoderite D. M. H,q,usnN. Schoderite, a new phosphovanadate mineral from Nevada. Bull,. Geol. Soc.Am.,7l, 1883 (1960) (abstract). The mineral occurs as yellowish-orange microcrystalline coatings associated with wavellite and vashegyite along fractures in phosphatic chert near Eureka, Nevada. It is in microscopic monoclinic, bladed tabular crystals, elongated parallel to (001). Unit cell dimensionsa 11.4, b 15.8, c 9.2 A., beta 79'. The strongest *-ray lines are 2.79-1O,9.6-10, 11.1-5, 15.8 A.-4. It is biaxial, positive, alpha 1.542,beta 1.548,gamma 1.566, 2V (calcd) 60", 'y:6. Formula 2AlrO3'&O5'VrO6'16H2O. At room temperature in a dry atmosphere, the mineral dehydrates to metaschoderite, 2AhOs'VrOs'&O6'12HrO. This rehydrates to schoderite on contact with water. Meta- schoderite has the same a, c, ar'd beta as schoderite, but b shrinks to 14.9 A. Biaxial, posi- tive (given as negative),a 1.598,p 1.604,"y 1626,2V 73'(calcd.),2:b.The strongest *-ray line are 7.5-10, 14.9-6, 11.1-5, 3.02 A.-3. The name is for William P. Schoder, research chemist, Union Carbide Nuclear Co. Drscussrox.-Presumably to be classed with the aluminum vanadates steigerite and al'vanite. Mrcnarl Fr,rrscnrn Unnamed Pt and Pd minerals L B. Bonovsr<rr, A. N. Dtnv, m+o L D. Mnncrrurovn. Application of the method of local r-ray spectrographic analysis to the study of minerals of the platinum grot::p. GeoI. -

Tnn Americnx Mineralogist of AMERTCA JOTIRNAL of the MTNERAIOGTCAT-Soctety
Tnn AMERIcnx MINERALoGIST oF AMERTCA JOTIRNAL OF THE MTNERAIOGTCAT-SOCTETy Vol. 55 MAY-JUNE Nos. 5 and 6 KINOITE, A NEW HYDROUS COPPER CALCIUM SILICATE MINERAL FROM ARIZONA of Arizona, Jonu W. ANruoNv, DepartmentoJ Geology,University Tucson,Arizona 85721 AND Rosrnr B. LaucnoN, I{ ASA, Geology Branch, Monneil Spacecraft Center, Houslon, Teras 77058. Assrnlcr Kinoite, cuzcarsrsoro.2H2o,a new speciesfrom the northern santa Rita Mountains, Pima county, Arizona, occursas singlecrystals and in veinlets associatedwith apophyl- lite, native copper, and copper sulfide minerals. It was found in drill coreswhich cut skarn developedin a Paleozoiclimestone sequence. X-ray crystallographicdata include:o:6.990+.004, b:12"89O+ '003,c:5'654* '002 A;F:96'05'+O+t;V:507.@7 At; .puc" group,P21fm.Strongestpowderdiffractionlines are4.72(10),3.052 (8),6.Mr (z),i.io 1+1,s.rss (3),2.9s1 (3), 2.315 (3), and 2.078 Q) L It is monoclinicwith a:b:c:.542:l:,.439, and B:96o95'. Crystalmorphology is domi- presental- nated by lhk\\, ail. crystals are tabular in the b-c plane. {011} is commonly though terminal forms are poorly developed.It exhibits excellent [010] cleavageand distinct [100] and [001] cleavages.It is deepazurite blue and transparentto translucent. Measureddensity is 3.16+.03;the calculatedvalue is 3.193g/cr,rll with Z:2. Hardnessis about 5 on the Mohs scale.crystals are optically(-);2v:68'; a:1.638 (iralegreenish : (a blue),B : 1.665(blue), 7 : 1.676(deep blue). Z /p neatoo, X 6,r distinct' The mineral is named for Fr. -

Minerals Localities
MINERALS and their LOCALITIES This book is respectfully dedicated to the memory of Dr. John Sinkankas for his kind initiative and support to publish this book in English version. MINERALS and their LOCALITIES Jan H. Bernard and Jaroslav Hyršl Edited by Vandall T. King © 2004 by Granit, s.r.o. © 2004 Text by Jan H. Bernard and Jaroslav Hyršl © 2004 Photos by Jaroslav Hyršl (463), Studio Granit (534), Jaromír Tvrdý (34), Petr Zajíček (4) The photographed specimens are from the collections of both authors as well as from many other collections. The autors are grateful to all institutions and persons who allowed to photograph their specimens for this book. Front cover photos: Turquoise, polished, 55 mm, Zhilandy, Kazakhstan, G Galena, 45 mm, Madan, Bulgaria, G Sphalerite, xx 12 mm, Morococha, Peru, H Gypsum, xx 40 mm, Las Salinas, Peru, H Variscite, xx 5 mm, Itumbiara, Brazil, H Rhodochrosite, polished, 50 mm, Capillitas, Argentina, H Back cover photo: Wolframite, 45 mm, Yaogangxian, China, H Page 1: Muscovite, 45 mm, Linopolis, Brazil, H Page 2: Vivianite, 100 mm, Huanzala, Bolivia, H Page 3: Liddicoatite, polished, 70 mm, Anjanabonoina, Madagaskar, G Page 5: Opal - fire, polished, 50 mm, Mezezo, Ethiopia, G Page 12: Brazilianite, 35 mm, Linopolis, Brazil, H Page 13: Gold, 35 mm, Eagle's Nest Mine, California, G Published by Granit, s.r.o. Štefánikova 43, 150 00 Praha 5, Czech Republic e-mail: [email protected] www.granit-publishing.cz Composition and reproduction by Studio VVG, Prague Printed in Czech Republic by Finidr, s.r.o., Český Těšín 14/02/03/01 All rights reserved. -

Spring 2003 Gems & Gemology
Spring 2003 VOLUME 39, NO. 1 EDITORIAL 1 In Honor of Dr. Edward J. Gübelin Alice S. Keller FEATURE ARTICLES ______________ 4 Photomicrography for Gemologists John I. Koivula Reviews the fundamentals of gemological photomicrography and introduces new techniques, advances, and discoveries in the field. pg. 16 NOTES AND NEW TECHNIQUES ________ 24 Poudretteite: A Rare Gem Species from the Mogok Valley Christopher P. Smith, George Bosshart, Stefan Graeser, Henry Hänni, Detlef Günther, Kathrin Hametner, and Edward J. Gübelin Complete description of a faceted 3 ct specimen of the rare mineral poudretteite, previously known only as tiny crystals from Canada. 32 The First Transparent Faceted Grandidierite, from Sri Lanka Karl Schmetzer, Murray Burford, Lore Kiefert, and Heinz-Jürgen Bernhardt Presents the gemological, chemical, and spectroscopic properties of the first known transparent faceted grandidierite. pg. 25 REGULAR FEATURES __________________________________ 38 Gem Trade Lab Notes • Diamond with fracture filling to alter color • Intensely colored type IIa diamond with substantial nitrogen-related defects • Diamond with unusual overgrowth • Euclase specimen, with apatite and feldspar • “Cherry quartz” glass imitation • Cat’s-eye opal • “Blue” quartz • Heat-treated ruby with a large glass-filled cavity • Play-of-color zircon 48 Gem News International • 2003 Tucson report • Dyed “landscape” agate • Carved Brazilian bicolored beryl and Nigerian tourmaline • New deep pink Cs-“beryl” from Madagascar • New demantoid find in Kladovka, Russia • Fire opal from Oregon pg. 33 • Cultured pearls with diamond insets • Gemewizard‰ gem communication and trading software • AGTA corundum panel • European Commission approves De Beers Supplier of Choice initiative • Type IaB diamond showing “tatami” strain pattern • Poldervaartite from South Africa • Triphylite inclusions in quartz • LifeGem synthetic diamonds • Conference reports • Announcements 65 The Dr. -
Crystal's Rock
Pretoria Gem and Mineral Club Affiliated to the Federation of Southern African Gem and Mineralogical Societies July 2010 Pretoria Rockhound Digest Pretoria Gem and Mineral Club Clubhouse: P.O. Box 36 888 No. 29, 26th Street, Menlo Park, Pretoria Menlo Park 0102 Tel. 012 – 460 1494 (only Sat. 11:00 – 15:00) South Africa website: www.pgmc.co.za Chairman: Lex Krabbendam, 083 449 6812 Treasurer: Dave de Villiers [email protected] Secretary: Di de Villiers 082 562 8983 Vice-Chairman: Dieter Heinichen 012-991 5839 Editor : Heidi Naudé, 072 923 2180 [email protected] Editor: [email protected] Programme Highlights for July 2010 *** Silversmithing Course: Ring with dark groove around stone 1 July *** Mineral Meeting: Quartz Specimens 3 July *** Swop & Sell and Social 3 July *** Silversmithing Course: Plain Ring Chain 17 July *** Silversmithing Course: Wire Spiral Pendant 22 July A very warm welcome to all the new members who joined recently: Marie Schoeman, André & Mathew Rossouw and Johann Deetlefs. May your association with the Club be enjoyable, long and productive. Page 2 PGMC Programme for July 2010: (as 24.06.2010) Date Event Time Contact Details 1 July Silversmithing Course: 18:30 – 21:00 Bernard Strydom, 078 187 3267 Ring with dark groove around stone 3 July Mineral Meeting: Quartz Specimens 12:00 – 13:00 Piet Reitz, 012 – 998 5452 Swop & Sell and Social at the Clubhouse 13:00 – 15:00 Lex Krabbendam, 083 449 6812 Lapidary and Silversmithing Workshops open 8 July Silversmithing Workshop open 18:30 – 21:00 Bernard Strydom, 078 -
Mineral Formation on Metallic Copper in Afuture Repository Site Environment
SKI Report 96:38 Mineral Formation on Metallic Copper in a "Future Repository Site Environment" Örjan Amcoff Katalin Holényi April 1996 ISSN 1104-1374 ISRN SKI-R--96/38--SE ffiMM^ STATENS KÄRNKRAFTINSPEKTION VOL 2 7 fia 1 5 Swedish Nuclear Power Inspectorate SKI Report 96:38 Mineral Formation on Metallic Copper in a "Future Repository Site Environment" Örjan Amcoff Katalin Holényi University of Uppsala Institute of Earth Sciences, Mineralogy-Petrology, Norbyvägen 18B, 752 36 Uppsala, Sweden April 1996 This report concerns a study which has been conducted for the Swedish Nuclear Power Inspectorate (SKI). The conclusions and viewpoints presented in the report are those of the authors and do not necessarily coincide with those of the SKI. NORSTEDTS TRYCKERI AB Stockholm 1996 SUMMARY. Formation of copper minerals in a "future repository site environment" is discussed. Since reducing conditions are expected much effort has been concentrated on Cu-sulphides and CuFe-sulphides. However, oxidizing conditions are also discussed. A list of copper minerals are included. It is concluded that mineral formation and mineral transitions on the copper canister surface will be governed by kinetics and metastabilities rather than by stability relations. The sulphides formed are less likely to form a passivating layer, and the rate of sulphide growth will probably be governed by the rate of transport of reacting species to the cansiter surface. A series of tests are recommended, in a milieu resembling the initial repository site conditions. SAMMANFATTNING. Bildning av kopparmineral i en framtida slutförvarsmiljö diskuteras. Eftersom reducerande betingelser kan förväntas har Cu-sulfider och CuFe-sulfider getts stor plats, men även oxiderande betingelser diskuteras. -
Volume 24 / No. 1 / 1994
Volume 24 No. 1. January 1994 The Journal of Gemmology The Gemmological Association and Gem Testing Laboratory of Great Britain OFFICERS AND COUNCIL Past Presidents: Sir Henry Miers, MA, D.Sc, FRS Sir William Bragg, OM, KBE, FRS Dr. G.F. Herbert Smith, CBE, MA, D.Sc. Sir Lawrence Bragg, CH, OBE, MC, B.Sc, FRS Sir Frank Claringbull, Ph.D., F.Inst.P., FGS Vice-Presidents: R. K. Mitchell A.E. Farn D.G. Kent E. M. Bruton Council of Management C.R. Cavey T.J. Davidson N.W. Deeks E.C. Emms I. Thomson V.P. Watson R.R. Harding Members' Council A. J. Allnutt J. Kessler R. Shepherd P. J. E.Daly G. Monnickendam R. Velden P. Dwyer-Hickey L. Music D. Warren R. Fuller, J.B. Nelson CH. Winter B. Jackson P. G. Read G.H. Jones I. Roberts Branch Chairmen: Midlands Branch: D.M. Larcher, North-West Branch: I. Knight Examiners: A. J. Allnutt, M.Sc, Ph.D., FGA G. H. Jones, B.Sc, Ph.D., FGA L. Bartlett, B.Sc, M.Phil., FGA, DGA D. G. Kent, FGA E. M. Bruton, FGA, DGA R. D. Ross, B.Sc, FGA C. R. Cavey, FGA P. Sadler, B.Sc, FGS, FGA, DGA S. Coelho, B.Sc, FGA, DGA E. Stern, FGA, DGA A.T. Collins, B.Sc, Ph.D Prof. I.Sunagawa, D.Sc. B. Jackson, FGA M. Tilley, GG, FGA E. A. Jobbins, B.Sc, C. Eng., FIMM, FGA C. Woodward, B.Sc, FGA, DGA Editor: E. A. Jobbins Production Editor: Mary A. Burland The Gemmological Association and Gem Testing Laboratory of Great Britain 27 Greville Street, London EC1N 8SU Telephone: 071-404 3334 Fax: 071-404 8843 TheJournal of Gemmology VOLUME 24 NUMBER ONE JANUARY 1994 Cover Picture Ruby crystals from Mong Hsu, Myanmar (Burma): the terminated crystal (9mm high) is not heat treated.