Monitoring of Fluoride Concentration in Groundwater of Tosham Block, Bhiwani District, NW, India: Correlation with Physico-Chemical Parameters
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

District Wise Skill Gap Study for the State of Haryana.Pdf
District wise skill gap study for the State of Haryana Contents 1 Report Structure 4 2 Acknowledgement 5 3 Study Objectives 6 4 Approach and Methodology 7 5 Growth of Human Capital in Haryana 16 6 Labour Force Distribution in the State 45 7 Estimated labour force composition in 2017 & 2022 48 8 Migration Situation in the State 51 9 Incremental Manpower Requirements 53 10 Human Resource Development 61 11 Skill Training through Government Endowments 69 12 Estimated Training Capacity Gap in Haryana 71 13 Youth Aspirations in Haryana 74 14 Institutional Challenges in Skill Development 78 15 Workforce Related Issues faced by the industry 80 16 Institutional Recommendations for Skill Development in the State 81 17 District Wise Skill Gap Assessment 87 17.1. Skill Gap Assessment of Ambala District 87 17.2. Skill Gap Assessment of Bhiwani District 101 17.3. Skill Gap Assessment of Fatehabad District 115 17.4. Skill Gap Assessment of Faridabad District 129 2 17.5. Skill Gap Assessment of Gurgaon District 143 17.6. Skill Gap Assessment of Hisar District 158 17.7. Skill Gap Assessment of Jhajjar District 172 17.8. Skill Gap Assessment of Jind District 186 17.9. Skill Gap Assessment of Kaithal District 199 17.10. Skill Gap Assessment of Karnal District 213 17.11. Skill Gap Assessment of Kurukshetra District 227 17.12. Skill Gap Assessment of Mahendragarh District 242 17.13. Skill Gap Assessment of Mewat District 255 17.14. Skill Gap Assessment of Palwal District 268 17.15. Skill Gap Assessment of Panchkula District 280 17.16. -

Vegetation Indices Mapping for Bhiwani District of Haryana (India) Through LANDSAT-7ETM+ and Remote Sensing Techniques
AL SC R IEN TU C A E N F D O N U A N D D A E I T L Journal of Applied and Natural Science 7 (2): 874 - 879 (2015) I O P N P JANS A ANSF 2008 Vegetation indices mapping for Bhiwani district of Haryana (India) through LANDSAT-7ETM+ and remote sensing techniques A.Bala 1,3 , K.S.Rawat 2, A. Misra 3, A. Srivastava 3 1Department of Civil Engineering, World College of Technology & Management, Gurgaon, INDIA 2Centre for Remote Sensing and Geo-Informatics, Sathyabama University, Chennai-(T.N.), INDIA 3Department of Civil Engineering, The Northcap University (Formerly ITM University), Gurgaon, INDIA *Corresponding author. E-mail: [email protected] Received: November 18, 2014; Revised received: September 20, 2015; Accepted: October 28, 2015 Abstract: This study describes the VIs Vegetation Condition Index in term of vegetation health of wheat crop; with help of LANDSAT-7ETM+ data based NDVI and LAI for Bhiwani District of Haryana states (India) and gave the spatial development pattern of wheat crop in year 2005 over the study area of India. NDVI is found to vary from 0.3 to 0.8. In northern and southern parts of study area NDVI varied from 0.6 to 0.7 but in western part of Bhiwani showed NDVI 0.2 to 0.4 due to fertility of soil and well canal destitution. LAI showed variation from 1 to 6 according to the health of crop as the same manner of NDVI because LAI VI is NDVI dependent only change the manner of representation of vegetation health, due to this fact relation curve (r 2=) between NDVI and LAI of four different grow- ing date of sates are in successively increasing order 0.509, 0.563, 0.577 and 0.719. -

Village & Townwise Primary Census Abstract
CENSUS OF INDIA 1991 SERIES -8 HARYANA DISTRICT CENSUS HANDBOOK PART XII-A&B VILLAGE, & TOWN DIRECTORY VILLAGE & TOWNWISE PRIMARY CENSUS ABSTRACT DIST.RICT BHIWANI Director of Census Operations Haryana Published by : The Government of Haryana, 1995 , . '. HARYANA C.D. BLOCKS DISTRICT BHIWANI A BAWAN I KHERA R Km 5 0 5 10 15 20 Km \ 5 A hAd k--------d \1 ~~ BH IWANI t-------------d Po B ." '0 ~3 C T :3 C DADRI-I R 0 DADRI - Il \ E BADHRA ... LOHARU ('l TOSHAM H 51WANI A_ RF"~"o ''''' • .)' Igorf) •• ,. RS Western Yamuna Cana L . WY. c. ·......,··L -<I C.D. BLOCK BOUNDARY EXCLUDES STATUtORY TOWN (S) BOUNDARIES ARE UPDATED UPTO 1 ,1. 1990 BOUNDARY , STAT E ... -,"p_-,,_.. _" Km 10 0 10 11m DI';,T RI CT .. L_..j__.J TAHSIL ... C. D . BLOCK ... .. ~ . _r" ~ V-..J" HEADQUARTERS : DISTRICT : TAHSIL: C D.BLOCK .. @:© : 0 \ t, TAH SIL ~ NHIO .Y'-"\ {~ .'?!';W A N I KHERA\ NATIONAL HIGHWAY .. (' ."C'........ 1 ...-'~ ....... SH20 STATE HIGHWAY ., t TAHSil '1 TAH SIL l ,~( l "1 S,WANI ~ T05HAM ·" TAH S~L j".... IMPORTANT METALLED ROAD .. '\ <' .i j BH IWAN I I '-. • r-...... ~ " (' .J' ( RAILWAY LINE WIT H STA110N, BROAD GAUGE . , \ (/ .-At"'..!' \.., METRE GAUGE · . · l )TAHSIL ".l.._../ ' . '1 1,,1"11,: '(LOHARU/ TAH SIL OAORI r "\;') CANAL .. · .. ....... .. '" . .. Pur '\ I...... .( VILLAGE HAVING 5000AND ABOVE POPULATION WITH NAME ..,." y., • " '- . ~ :"''_'';.q URBAN AREA WITH POPULATION SIZE- CLASS l.ltI.IV&V ._.; ~ , POST AND TELEGRAPH OFFICE ... .. .....PTO " [iii [I] DEGREE COLLE GE AND TECHNICAL INSTITUTION.. '" BOUNDARY . STATE REST HOuSE .TRAVELLERS BUNGALOW AND CANAL: BUNGALOW RH.TB .CB DISTRICT Other villages having PTO/RH/TB/CB elc. -

Sr. No . Name of Municipality Name & Designastion of R.O
LIST OF RETURNING OFFICERS AND ASSISTANT RETURNING OFFICER FOR MUNICIPAL GENERAL ELECTIONS, 2018 SR. NAME OF NAME & DESIGNASTION WARD NO. MOBILE NO. EMAIL ADDRESS NO MUNICIPALITY OF R.O./A.R.O. /CONTACT NO. 1 2 3 4 5 6 1. M.C. Hathin Sh. Munish Sharma, IAS, Sub - 9650746944 (Palwal) Divisional Officer (C ), Hathin – 8368733455 cum-Returning Officer. 2. M.C. Hathin Sh. Naresh Kumar Jowel, - 0971194142 (Palwal) Tehsildar , Palwal -cum-A.R.O. Hathin. 3. M.C. Neelokheri Sh. Narinder Pal Malik, HCS - 90344-33168 (Karnal ) Sub Divisional Officer (C ) Karnal-cum-Returning Officer 4. M.C. Neelokheri Sh. Anand Kumar, Naib - 9416355344 (Karnal) Tehsildar, Neelokheri-cum- A.R.O. Neelokheri 5. M.C. Indri (Karnal) Sh. Pardeep Kaushick, HCS, - 9992056709 Sub Divisional Officer, Indri- cum-Returning Officer, Indri 6. M.C. Indri(Karnal) Sh. Hoshiyar Singh, Naib - 9416094972 Tehsildar, Indri-cum-A.R.O. Indri. 7. M.C. Assandh Sh. Anurag Dhalia, HCS - 9354534915 (Karnal) Sub Divisional Officer (C ) Assandh-cum-R.O. Assandh 8. M.C. Assandh Sh. Himmat Singh, Naib - 9466317051 (Karnal) Tehsildar, Assandh-cum- A.R.O. Assandh. 9. M.C. Kalayat Sh. Jagdeep Singh, HCS, - 08199851695 (Kaithal) SDO (C ), Kalayat-cum-R.O. Kalayat 10. M.C. Kalayat Sh. Raj Kumar, BDPO, - 9416347755 (Kaithal) Kalayat-cum-A.R.O. Kalayat. 11. M.C. Kanina Sh. Sandeep Singh, HCS, - 9416022388 (Mahendergarh) SDO © Kanina-cum-R.O. Kanina. 12. M.C. Kanina Sh. Om Parkash, BDPO, - - (Mahendergarh) Kanina -cum-A.R.O. Kanina. 13. M.C. Ateli Mandi Sh. Jagdish Sharma, HCS - 9416527818 (Mahendergarh) SDO (C ), Narnaul-cum-R.O. -
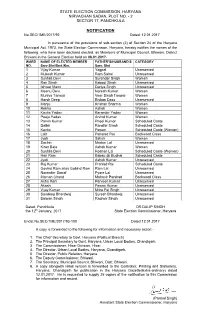
State Election Commission, Haryana Nirvachan Sadan, Plot No
STATE ELECTION COMMISSION, HARYANA NIRVACHAN SADAN, PLOT NO. - 2 SECTOR 17, PANCHKULA NOTIFICATION No.SEC/1ME/2017/92 Dated: 12.01.2017 In pursuance of the provisions of sub-section (2) of Section 24 of the Haryana Municipal Act, 1973, the State Election Commission, Haryana, hereby notifies the names of the following, who have been declared elected, as Members of Municipal Council, Bhiwani, District Bhiwani in the General Election held on 08.01.2017- WARD NAME OF ELECTED MEMBER FATHER’S/HUSBAND’S CATEGORY NO. Sarv.Shri/Smt./Km. Sarv. Shri 1 Vijay Kumar Yagpal Unreserved 2 Mukesh Kumar Ram Sahai Unreserved 3 Sushila Devi Surender Singh Women 4 Ran Singh Kabool Singh Unreserved 5 Ishwar Mann Dariya Singh Unreserved 6 Meenu Devi Naresh Kumar Women 7 Muniya Tanwar Veer Singh Tanwar Women 8 Harsh Deep Bishan Dass Unreserved 9 Manju Krishan Sharma Women 10 Rekha Ashok Women 11 Kavita Yadav Karambir Yadav Women 12 Pooja Yadav Arvind Kumar Women 13 Parvin Kumar Phool Kumar Scheduled Caste 14 Dalbir Randhir Singh Scheduled Caste 15 Kavita Pawan Scheduled Caste (Women) 16 Lalit Pahalad Rai Backward Class 17 Jyoti Satish Women 18 Sachin Madan Lal Unreserved 19 Kiran Bala Ashok Kumar Women 20 Sushila Devi Roshan Lal Scheduled Caste (Women) 21 Hari Ram Babau @ Budhai Scheduled Caste 22 Jyoti Ashok Kumar Unreserved 23 Raj Kumar Prahlad Rai Scheduled Caste 24 Govind Ram alias Gobind Ram Ram Lal Unreserved 25 Narender Sarraf Pyare Lal Unreserved 26 Maman Chand Mahavir Parshad Backward Class 27 Asha Rani Parveen Kumar Unreserved 28 Akash Pawan Kumar Unreserved 29 Vijay Kumar Mitra Pal Singh Unreserved 30 Sandeep Bhardwaj Suresh Bhardwaj Unreserved 31 Balwan Singh Raghvir Singh Unreserved Dated, Panchkula DR.DALIP SINGH the 12th January, 2017 State Election Commissioner, Haryana Endst.No.SEC/1ME/2017/93-100 Dated:12.01.2017 A copy is forwarded to the following for information and necessary action:- 1. -
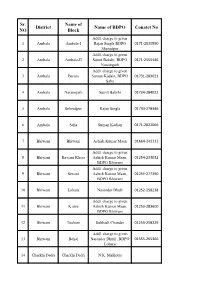
Sr. NO District Name of Block Name of BDPO Conatct No
Sr. Name of District Name of BDPO Conatct No NO Block Addl. charge to given 1 Ambala Ambala-I Rajan Singla BDPO 0171-2530550 Shazadpur Addl. charge to given 2 Ambala Ambala-II Sumit Bakshi, BDPO 0171-2555446 Naraingarh Addl. charge to given 3 Ambala Barara Suman Kadain, BDPO 01731-283021 Saha 4 Ambala Naraingarh Sumit Bakshi 01734-284022 5 Ambala Sehzadpur Rajan Singla 01734-278346 6 Ambala Saha Suman Kadian 0171-2822066 7 Bhiwani Bhiwani Ashish Kumar Maan 01664-242212 Addl. charge to given 8 Bhiwani Bawani Khera Ashish Kumar Maan, 01254-233032 BDPO Bhiwani Addl. charge to given 9 Bhiwani Siwani Ashish Kumar Maan, 01255-277390 BDPO Bhiwani 10 Bhiwani Loharu Narender Dhull 01252-258238 Addl. charge to given 11 Bhiwani K airu Ashish Kumar Maan, 01253-283600 BDPO Bhiwani 12 Bhiwani Tosham Subhash Chander 01253-258229 Addl. charge to given 13 Bhiwani Behal Narender Dhull , BDPO 01555-265366 Loharu 14 Charkhi Dadri Charkhi Dadri N.K. Malhotra Addl. charge to given 15 Charkhi Dadri Bond Narender Singh, BDPO 01252-220071 Charkhi Dadri Addl. charge to given 16 Charkhi Dadri Jhoju Ashok Kumar Chikara, 01250-220053 BDPO Badhra 17 Charkhi Dadri Badhra Jitender Kumar 01252-253295 18 Faridabad Faridabad Pardeep -I (ESM) 0129-4077237 19 Faridabad Ballabgarh Pooja Sharma 0129-2242244 Addl. charge to given 20 Faridabad Tigaon Pardeep-I, BDPO 9991188187/land line not av Faridabad Addl. charge to given 21 Faridabad Prithla Pooja Sharma, BDPO 01275-262386 Ballabgarh 22 Fatehabad Fatehabad Sombir 01667-220018 Addl. charge to given 23 Fatehabad Ratia Ravinder Kumar, BDPO 01697-250052 Bhuna 24 Fatehabad Tohana Narender Singh 01692-230064 Addl. -

List of SARAL Kendra
Address of Mobile Antyodaya Designation Name of number of Bhawan/SARAL Link to the Link to Location Type of of officer in- Officer in- Officer-in- Kendra/Antyoday Location Name on Location on Photos of ambala Location Type Centre charge charge charge a SARAL Kendra Google Maps Google Maps the centre Mini Secretariat, Near Judicial https://goo.g SARAL Meenaksh Complex, Ambala SARAL Kendra - l/maps/DAp Ambala Ambala HQ Kendra SDM i Dahiya 7508169999 City Ambala Vz8WdbFz Surjit Rajeev Gandhi Antyodaya Kaur(Addl. Seva Kendra, near Antyodaya Bhawan - Ambala Ambala HQ Bhawan DSWO Charge) 9896042142 BDO block Ambala NA NA Mini Secretariat, Ground floor, SDM Antyodaya Office, Sarakpur https://goo.g Subdivisio SARAL Girish Road, Barara- Antyodaya SARAL l/maps/kritw Ambala Barara n Kendra SDM Kumar 9813028999 133201 Kendra - Barara tVpWrR2 CEO Zila Parishad, Mini Secretariart, https://goo.g Antyodaya Smt. Aditi Ground floor, l/maps/UMH Naraingar Subdivisio SARAL (Addl. Naraingarh- Antyodaya SARAL MrMWaBUN Ambala h n Kendra SDM Charge) 9.897E+09 134203 Kendra - Naraingarh 2 Mini Secretariat, Ground floor, SDM Office, Ambala Cantonment, Near Antyodaya Ambala Cantt Bus Antyodaya SARAL https://goo.g Ambala Subdivisio SARAL Subhash Stand, Ambala Kendra - Ambala l/maps/Nwp Ambala Cantt n Kendra SDM Sihag 8199830003 Cantt. Cantt G4H22qV12 https://dri ve.google. com/open ?id=1hIXz Saral kendra, DC _QMtrAyY office, Mini https://goo.g LD6PaPY1 SARAL Satish secretariat , SARAL Kendra - l/maps/HXAY vp1tMxLp Bhiwani Bhiwani HQ Kendra SDM Kumar 8901245645 Bhiwani Bhiwani 9kBm27r d4eg Working women's hostel, Bhim Antyodaya Amit stadium road, Antyodaya Bhawan - Bhiwani Bhiwani HQ Bhawan DSWO Sharma 7015975523 Bhiwani Bhiwani NA https://dri ve.google. -

Distribution of Fluoride in Groundwater in Bhiwani District and Its Suitability Assessment for Drinking Purpose
International Journal of Engineering Research & Technology (IJERT) ISSN: 2278-0181 Vol. 3 Issue 4, April - 2014 Distribution of Fluoride in Groundwater in Bhiwani District and its Suitability Assessment for Drinking Purpose Manjeet*,1, B. P. Singh#,2, J. K. Sharma $,3 *Doctorial Research Scholar, #Dean Academic Affairs, $$Pro-vice Chancellor, DCRUST, Murthal, Haryana, India DCRUST, Murthal, Haryana, Ind ia JECRC University, Jaipur, India Abstract— Groundwater samples collected either from the bore- actions with minimal negative impacts on population health. wells (forms a part of municipal water supply) or from the hand In the present study, groundwater of Bhiwani District was pumps (direct consumption) were analyzed for fluoride in mapped for fluoride distribution, which is mainly used for Bhiwani District (India). The results indicate considerable drinking and other domestic purposes. variations among the analyzed groundwater samples and the concentration of fluoride ranged from 1.1 to 2.1 mg/l. In most of the groundwater samples the concentration of fluoride was found Experimental to be moderately higher, when compared to WHO standard for drinking water, which may leads to the associated health risks in Site specifications and sampling: The district occupies an area urban population, if the groundwater is being used without of 5,140 square kilometres (1,980 sq mi). It is situated proper treatment. Further it is suggested that the sources of between 28.19 deg. & 29.05 deg. north latitudes and 75.26 deg. municipal water supply must be established in a region where an and 76.28 deg. east longitudes. It has 444 villages with a adequate level of fluoride was observed. -

Bhiwani, One of the Eleven Districts! of Haryana State, Came Into Existence
Bhiwani , one of the eleven districts! of Haryana State , came into existence on December 22, 1972, and was formally inaugurated on Ja ilUary 14 , 1973. It is mmed after the headquarters . town of Bhiwani , believed to be a corruption of the word Bhani. From Bhani, it changed to Bhiani and then Bhiwani. Tradi tion has it that one Neem , a Jatu Rajput , who belonged to vill age B:twani 2, then in Hansi tahsil of the Hisar (Hissar) di strict , came to settle at Kaunt , a village near the present town of Bhiwani. Thi s was re sen ted by the local Jat inhabitants, and they pl otted his murder. Neem was war ned by a Jat woman , named Bahni, and thus forewarned , had his revenge on th e loc al Jat s. He killed m~st of them at a banquet, the site of which wa s min ed with gun- powder. He m'lrried B:thni and founded a village nam ed after her. At the beginning of the nineteenth century , Bhiwani was an in signifi cant village in the Dadri pargana, under the control of the Nawab of Jhajj ar. It is, how - ever, referred to as a town when the British occupied it in 1810 .3 It gained importance during British rule when in 1817, it was sel ected for the site of a mandi or free market, and Charkhi Dadri, still under the Nawa bs, lost its importance as a seat of commerce. Location and boundaries.- The district of Bhiwani lie s in be twee n latitude 2&0 19' and 290 OS' and longitude 750 28' to 760 28' . -

Haryana Chapter Kurukshetra
Panchkula Yamunanagar INTACH Ambala Haryana Chapter Kurukshetra Kaithal Karnal Sirsa Fatehabad Jind Panipat Hisar Sonipat Rohtak Bhiwani Jhajjar Gurgaon Mahendragarh Rewari Palwal Mewat Faridabad 4 Message from Chairman, INTACH 08 Ambala Maj. Gen. L.K. Gupta AVSM (Retd.) 10 Faridabad-Palwal 5 Message from Chairperson, INTACH Haryana Chapter 11 Gurgaon Mrs. Komal Anand 13 Kurukshetra 7 Message from State Convener, INTACH Haryana Chapter 15 Mahendragarh Dr. Shikha Jain 17 Rohtak 18 Rewari 19 Sonipat 21 Yamunanagar 22 Military Heritage of Haryana by Dr. Jagdish Parshad and Col. Atul Dev SPECIAL SECTION ON ARCHAEOLOGY AND RAKHIGARHI 26 Urban Harappans in Haryana: With special reference to Bhiwani, Hisar, Jhajjar, Jind, Karnal and Sirsa by Apurva Sinha 28 Rakhigarhi: Architectural Memory by Tapasya Samal and Piyush Das 33 Call for an International Museum & Research Center for Harrapan Civilization, at Rakhigarhi by Surbhi Gupta Tanga (Director, RASIKA: Art & Design) MESSAGE FROM THE CHAIRMAN INTACH Over 31 years from its inception, INTACH has been dedicated towards conservation of heritage, which has reflected in its various works in the field of documentation of tangible and intangible assets. It has also played a crucial role in generating awareness about the cultural heritage of the country, along with heritage awareness programmes for children, professionals and INTACH members. The success of INTACH is dedicated to its volunteers, conveners and members who have provided valuable inputs and worked in coordination with each other. INTACH has been successful in generating awareness among the local people by working closely with the local authorities, local community and also involving the youth. There has been active participation by people, with addition of new members every year. -

District Wise List of Registered and Un-Registered Gaushalas of Haryana State Alongwith Number of Animals Kept in the Gaushalas. AMBALA S.No
District wise List of Registered and Un-Registered Gaushalas of Haryana State alongwith number of Animals kept in the Gaushalas. AMBALA S.No. Distt. Name of Gaushala Haryana Regn. No. No. of Animals Sr. No. 1 1 Shri Rambag Gaushala, Rambag road, 607 412 Ambala Cantt. 2 2 Shri Kewal Krishan Miglani Gaushala 560 462 Samiti, Purani Gaas Mandi, Ambala City 3 3 Gaushala Trust Society,Spatu Road Ambala 1860 444 City 4 4 Gori Shanker Gau Raksha Samiti, Kalpi, 371 215 Near Power House, Saha, Distt. Ambala 5 5 Shri Govind Gaushala Samiti,Barara, Distt. 128 546 Ambala. 6 6 Shri Krishan Gaushala Samiti, Yamkeshwar 1035 939 Tirth Huseni, Naraingarh, Distt. Ambala. 7 7 Gurcharan Gaushalas Sanstha, Vill Bhunni, 40 25 P.O. Sonta, Distt. Ambala 8 8 Shri Radha Madhav Gaudham, Vill Mokha 642 94 Majra, Distt. Ambala. Total number of Animals 3137 BHIWANI 9 1 Shri Gaushala Trust, Bhiwani, Distt. 11 3890 Bhiwani. 10 2 Shri Shiv Dharmarth Gaushala, Dhuleri, 277 609 Distt. Bhiwani. 11 3 Shri Krishan Gaushala, Bamla 256 70 12 4 Shri Shyam Gaushala Trust, Mandana, 2800 234 Distt. Bhiwani. 13 5 Shri Rishi Kul Gaushala, Nimbriwali, Tehsil 40 277 & Distt. Bhiwani 14 6 Shri Gomath Gaushala, Leghan, Distt. 981 323 Bhiwani 15 7 Shri Shiv Muni Gaushala, Jitwawas, Post 436 488 office Leghan, Distt. Bhiwani 16 8 Shri Krishan Sudama Maitri Gaushala 3030 420 Society, V.P.O. Kharak Kalan , Distt. Bhiwani. 17 9 Shri Baba Dhuniwala Gau-Sewa Trust, 359 2803 Dinod, Distt. Bhiwani 18 10 Shri Krishan Gaushala,Tosham Road, 727 2205 Siwani Mandi, Distt. -

Lokayukta Haryana
LOKAYUKTA HARYANA ANNUAL REPORT FOR THE YEAR 2018-2019 (01.04.2018 TO 31.03.2019) After I had taken oath as Lokayukta of Haryana on 19th July, 2016, this is my 3rd Annual Report on the functioning of Lokayukta Institution for the aforesaid period under report being submitted to the Hon’ble Governor of Haryana as required under Section 17 (3) of the Haryana Lokayukta Act, 2002. Lokayuktas have been unearthing corruption cases, recommending measures to redress grievances of the people and above all, acting as a much needed safety valve to release the bottled up pressure of aggrieved citizens, which, if allowed to accumulate, would put a question mark on the credibility of our administrative apparatus. The very existence of a Lokayukta helps to generate a feeling of assurance among the public at large, that they have a mechanism to fall back upon when faced with corrupt public servants. Lokayukta investigates cases of corruption, where substantiated, recommend action. It is a great check on corruption, brings about transparency in the system, and makes administrative machinery citizen friendly. His functions largely depend upon jurisdiction vested in him and facilities provided 2 for taking cognizance of citizens’ grievances promptly, deftly and expeditiously through simple, informal mechanism devoid of technicalities. Corruption is internationally recognized a major problem, capable of endangering stability and security of society, threatening social, economic and political development and undermining the values of democracy and morality. It has assumed alarming proportions resultantly public funds going into private hands leading to enrichment of bribe givers and bribe takers. Corruption, inefficiency, delays and insensitivity to people’s grievances can be identified key problems besetting the nation.