Internal Anatomy and Physiology
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Star Fish Early Development Amphioxus Gastrulation
Faculty of Biological Science and Technology Zoology and Botanical department Practical embryology Amphibian development (4 mm larvae; sagittal sections) By: Shirin Kashfi Ph.D in Animal Development [email protected] tail bud stage At the time of neurulation termination, a part which is known as tail bud develops in the end of embryo body The entire body elongate and shorten in dorso-ventral direction in some extend First muscular responses to external stimuli develop, so this stage refer as muscular response stage too Tail bud is elongated and contains neural tube, notochord and unsegmented mesoderm as well as dorsal and ventral tail fin Five primary brain vesicles are developed (telencephalon, diencephalon, mesencephalon, metencephalon and myelencephalon) Retina (neural retina and retinal pigmented epithelium) and lens placode are formed Auditory vesicle in associated with hind brain and olfactory placode in associated with forebrain (telencephalon) are developed Heart rudiment is developed below to pharynx from mesoderm Foregut, midgut and hindgut are formed. In foregut oral cavity are expanded ventrally and laterally, pharynx and liver diverticulum can be recognizable Pronephric kidney is developed from intermediate mesoderm Somites are formed stage 18, 96 hpf, 4 mm in all sections please consider anterior, posterior, dorsal and ventral directions surface ectoderm yolky endoderm yolky endoderm can be seen in these sections gradually head region is appeared From: embryo/amphibian development/Book - The Frog Its Reproduction -

Embryology of Branchial Region
TRANSCRIPTIONS OF NARRATIONS FOR EMBRYOLOGY OF THE BRANCHIAL REGION Branchial Arch Development, slide 2 This is a very familiar picture - a median sagittal section of a four week embryo. I have actually done one thing correctly, I have eliminated the oropharyngeal membrane, which does disappear sometime during the fourth week of development. The cloacal membrane, as you know, doesn't disappear until the seventh week, and therefore it is still intact here, but unlabeled. But, I've labeled a couple of things not mentioned before. First of all, the most cranial part of the foregut, that is, the part that is cranial to the chest region, is called the pharynx. The part of the foregut in the chest region is called the esophagus; you probably knew that. And then, leading to the pharynx from the outside, is an ectodermal inpocketing, which is called the stomodeum. That originally led to the oropharyngeal membrane, but now that the oropharyngeal membrane is ruptured, the stomodeum is a pathway between the amniotic cavity and the lumen of the foregut. The stomodeum is going to become your oral cavity. Branchial Arch Development, slide 3 This is an actual picture of a four-week embryo. It's about 5mm crown-rump length. The stomodeum is labeled - that is the future oral cavity that leads to the pharynx through the ruptured oropharyngeal membrane. And I've also indicated these ridges separated by grooves that lie caudal to the stomodeum and cranial to the heart, which are called branchial arches. Now, if this is a four- week old embryo, clearly these things have developed during the fourth week, and I've never mentioned them before. -

Life Science Journal 2013;10(2) 479
Life Science Journal 2013;10(2) http://www.lifesciencesite.com Histological Observations on the Proventriculus and Duodenum of African Ostrich (Struthio Camelus) in Relation to Dietary Vitamin A. Fatimah A. Alhomaid1 and Hoda A. Ali2 1Dept. of Biology, Collage of Science and Arts, Qassim University, KSA 2Dept. of Nutrition and Food Science, Collage of Designs and Home Economy, Qassim University, KSA. Email: [email protected]; [email protected] Abstract: Research problem: The fine structure of the gut in different avian species in relation to dietary vitamins status have widely been studied with the exception of ostriches. Objectives: To study the histological structure of the African ostrich proventriculus and duodenum in relation to two levels of vitamin A (7500 and 9000) IU/kg diet using light microscope. Methods: Twenty male African ostriches with average age 65-67 weeks and apparently healthy were used. They were divided into two equal groups, the first one received diet adjusted to supply 7500 IU/kg diet vitamin A. The second group fed diet formulated to furnished 9000 IU/Kg diet vitamin A. Both diets were isonitrogenous and isocaloric. Body weight gain, feed intake and feed conversion rate were calculated. At the end of four weeks, pieces from the different parts of proventriculus and duodenum were taken for light microscopic examinations. Result: Body weight gain and feed conversion rate were improved in group received 9000IU of vitamin A comparable to group fed 7500IU. Histological structure of proventriculus of birds receiving 7500 IU/Kg vitamin A showing vascular congestion, thinning connective tissue and sloughing of the columnar epithelia lining the central collecting duct. -

2/2/2011 1 Development of Development of Endodermal
2/2/2011 ZOO 401- Embryology-Dr. Salah A. Martin DEVELOPMENT OF THE DIGESTIVE SYSTEM ◦ Primitive Gut Tube ◦ Proctodeum and Stomodeum ◦ Stomach Development of Endodermal Organs ◦ Duodenum ◦ Pancreas ◦ Liver and Biliary Apparatus ◦ Spleen ◦ Midgut Wednesday, February 02, 2011 DEVELOPMENT OF THE DIGESTIVE SYSTEM 2 Wednesday, February 02, 2011 Development of Ectodermal Organs 1 ZOO 401- Embryology-Dr. Salah A. Martin ZOO 401- Embryology-Dr. Salah A. Martin Primitive Gut Tube Proctodeum and Stomodeum The primitive gut tube is derived from the dorsal part of the yolk sac , which is incorporated into the body of The proctodeum (anal pit) is the primordial the embryo during folding of the embryo during the fourth week. anus , and the stomodeum is the primordial The primitive gut tube is divided into three sections. mouth . The epithelium of and the parenchyma of In both of these areas ectoderm is in direct glands associated with the digestive tract (e.g., liver and pancreas) are derived from endoderm . contact with endoderm without intervening The muscular walls of the digestive tract (lamina mesoderm, eventually leading to degeneration propria, muscularis mucosae, submucosa, muscularis of both tissue layers. Foregut, Esophagus. externa, adventitia and/or serosa) are derived from splanchnic mesoderm . The tracheoesophageal septum divides the During the solid stage of development the endoderm foregut into the esophagus and of the gut tube proliferates until the gut is a solid tube. trachea. information. A process of recanalization restores the lumen. Wednesday, February 02, 2011 Primitive Gut Tube 3 Wednesday, February 02, 2011 Proctodeum and Stomodeum 4 ZOO 401- Embryology-Dr. Salah A. -

Distribution of Digestive Enzymes in Cockroaches
Volume 24: Mini Workshops 311 Distribution of Digestive Enzymes in Cockroaches Flora Watson California State University, Stanislaus Department of Biological Sciences 801 W. Monte Vista Avenue Turlock, CA 95382 [email protected] Flora Watson is an Associate Professor in the Department of Biological Sciences at California State University, Stanislaus. She teaches lower and upper division Human and Animal Physiology courses at CSU, Stanislaus. Her research interest involves using immunohistochemistry to study the effects of cigarette smoke exposure on brain, lung and liver tissues of mice. Reprinted From: Watson, F. 2003. Distribution of digestive enzymes in cockroaches. Pages 311-316, in Tested studies for laboratory teaching, Volume 24 (M. A. O’Donnell, Editor). Proceedings of the 24th Workshop/Conference of the Association for Biology Laboratory Education (ABLE), 334 pages. - Copyright policy: http://www.zoo.utoronto.ca/able/volumes/copyright.htm Although the laboratory exercises in ABLE proceedings volumes have been tested and due consideration has been given to safety, individuals performing these exercises must assume all responsibility for risk. The Association for Biology Laboratory Education (ABLE) disclaims any liability with regards to safety in connection with the use of the exercises in its proceedings volumes. © 2003 Flora Watson Abstract The digestive tract of a cockroach is a tube modified into subdivisions, which serve specialized digestive functions: food reception, conduction and storage, internal digestion, absorption, conduction, and formation of feces. The three divisions of the cockroach digestive tract are the foregut, midgut, and the hindgut. The enzyme reaction in the digestive tract can be determined either by determining the amount of substrates (starch and proteins) in an enzyme-reaction mixture, or measuring the presence of product present. -

Insect Digestion
INSECT DIGESTION Zoo 514 Dr. Reem Alajmi Insect digestive system The alimentary canal is concerned with digestion and absorption of food stuffs. Different parts of the gut are concerned with different aspects of these functions. In fluid feeding insects digestion begin before the food is ingested through the injection or regurgitation of enzymes on to the food . In general it occurs in the mid gut (most of the enzymes are produced). The enzymes break down the complex substances in the food into simple substances which can be absorbed and assimilated. Enzymes function optimally only within limited range of pH and temperature. Absorption is passive process in some cases , but in others active transport occurs. Absorption of water is important in terrestrial insects , rectum remove water from faeces. • Insects of different groups consume astonishing variety of foods, including watery xylem sap, vertebrate blood, dry wood, bacteria and algae and the internal tissues of other insects. • Types of mouth parts correlates with the diets of insect. Also gut structure and function affected by nutrient composition of the food eaten by insect. • Insects that take solid food typically have a wide, straight, short gut with strong musculature and obvious protection from abrasion (especially in the midgut, which has no cuticular lining). • These features are most obvious in solid-feeders with rapid throughput of food, as in many insects and plant-feeding caterpillars In contrast, insects feeding on blood, sap or nectar usually have long, narrow, convoluted guts to allow maximal contact with the liquid food; here, protection from abrasion is unnecessary. The most obvious gut specialization of liquid-feeders is a mechanism for removing excess water to concentrate nutrient substances prior to digestion, as seen in hemipterans. -

Embryonic Development of the Chicken External Cloaca and Phallus
Scanning Electron Microscopy Volume 1986 Number 2 Article 35 5-23-1986 Embryonic Development of the Chicken External Cloaca and Phallus M. R. Bakst U. S. Department of Agriculture Follow this and additional works at: https://digitalcommons.usu.edu/electron Part of the Biology Commons Recommended Citation Bakst, M. R. (1986) "Embryonic Development of the Chicken External Cloaca and Phallus," Scanning Electron Microscopy: Vol. 1986 : No. 2 , Article 35. Available at: https://digitalcommons.usu.edu/electron/vol1986/iss2/35 This Article is brought to you for free and open access by the Western Dairy Center at DigitalCommons@USU. It has been accepted for inclusion in Scanning Electron Microscopy by an authorized administrator of DigitalCommons@USU. For more information, please contact [email protected]. SCANNING ELECTRON MICROSCOPY /1986/11 (Pages 653-659) SEM Inc., AMF O'Hare (Chicago), IL 60666-0507 USA EMBRYONIC DEVELOPMENT OF THE CHICKEN EXTERNAL CLOACA AND PHALLUS M. R. Bakst U.S. Department of Agriculture Agricultural Research Service Avian Physiology Laboratory Beltsville, Maryland 20705 Phone: 301 344 2545 (Received for publication March 22, 1986: revised paper received May 23, 1986) Abstract Introduction The use of the scanning electron microscope In the course of investigating the mechanisms has provided new detailed information about the of erection, ejaculation, and semen dilution in the embryonic development of the chicken external chicken, it was found necessary to determine the cloaca and phallus and has cor:sequently clarified the embryonic origin of the structures in the cloaca origin of the differences between the anatomy of the which form the chicken Phallus nonprotrudens chicken and turkey phallus. -

MINIREVIEW Posterior Gut Development in Drosophila: a Model System for Identifying Genes Controlling Epithelial Morphogen- Esis
Cell Research (1998), 8, 273-284 MINIREVIEW Posterior gut development in Drosophila: a model system for identifying genes controlling epithelial morphogen- esis LENGYEL JUDITH A* , XUE JUN LIU Department of Molecular, Cell and Developmental Biology University of California at Los Angeles, Los Angeles, CA USA, 90095-1606 USA ABSTRACT The posterior gut of the Drosophila embryo, consist- ing of hindgut and Malpighian tubules, provides a simple, well-defined system where it is possible to use a genetic approach to define components essential for epithelial mor- phogenesis. We review here the advantages of Drosophila as a model genetic organism, the morphogenesis of the ep- ithelial structures of the posterior gut, and what is known about the genetic requirements to form these structures. In overview, primordia are patterned by expression of hi- erarchies of transcription factors; this leads to localized expression of cell signaling molecules, and finally, to the least understood step: modulation of cell adhesion and cell shape. We describe approaches to identify additional genes that are required for morphogenesis of these simple epithelia, particularly those that might play a structural role by affecting cell adhesion and cell shape. Key words: Organogenesis, cell rearrangement, con- vergent extension, hindgut, Malpighian tubule. Advantages of Drosophila Work on Drosophila genetics began 90 years ago, when Thomas Hunt Morgan * Corresponding author: [email protected] Drosophila gut epithelial morphogenesis genes (who later received the Nobel Prize for his work) began studying inheritance in the fruit fly. At that time, the advantage of working with this small organism was that it reproduced rapidly in the laboratory, requiring only a simple growth medium, no special attention, and little expense. -
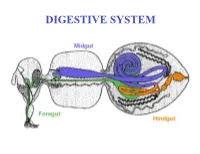
DIGESTIVE SYSTEM Generalized Insect Alimentary Tract the Digestive System Is Just a Tube Within a Surrounding Tube Called the Body
DIGESTIVE SYSTEM Generalized insect alimentary tract The digestive system is just a tube within a surrounding tube called the body. It starts with a mouth and ends with the anus. What goes on in between depends on the insect, its life stage and what it eats. The origin of the digestive tract. At the anterior pole of the embryo an indentation forms that will be the foregut or stomodeum. At the other end a similar thing occurs and the proctodeum or hindgut is formed. Both are lined by cuticle. They both are of ectodermal origin while the midgut is of mesodermal origin and is also called the mesenteron. This different origin of the midgut from the endoderm and not the ecotoderm probably explains why it is not lined with cuticle Anterior midgut invagination. In the bottom photo note the invagination starting forming the ventral furrow lumen (VF) MIDGUT FORMATION IN THE EMBRYO PMG in the above embryo shows the posterior midgut invagination cup where the posterior invagination shown in the drawing on the right will take place. Photo of Drosophila embryo. Hindgut invagination DIGESTIVE SYSTEM The digestive tract not only aids in obtaining, processing and digesting food molecules - It is the largest endocrine tissue in both humans and insects. The digestive system is involved in: 1. Obtaining food 2. Mechanically breaking it down into smaller particles that facilitate digestive enzymes acting on them 3. Enzymatic breakdown of larger food molecules into molecules that can pass through the digestive tract (midgut) and enter the hemolymph 4. Produces molecules or MESSENGERS (eg. -

Midgut/Hindgut Organs & Blood Supply
Midgut & Hindgut Organs & Their Blood Supply Lab 2 January 12, 2021 - Dr. Doroudi ([email protected]) Objectives: • Identify and name branches of the superior mesenteric artery • Identify and name branches of the inferior mesenteric artery • Identify the portal vein and its tributaries • Identify different parts of midgut and hindgut derivatives • Describe the innervation of the organs derived from the midgut and hindgut These are the relevant videos Watch this dissection guide video: covering the lab objectives: Volume 5 - The Internal Organs Identify checklist structures on the interactive photo and specimens in the virtual lab: The Abdominal Organs 5.2.9 Jejuno-ileum 5.2.10 Cecum and appendix 5.2.11 Wall of the colon 5.2.12 Colon 5.2.24 Arteries of the abdominal organs 5.2.25 Veins of the abdominal organs Viscera: Small intestine - Jejunum - Ileum - Ileocecal junction and valve - Identify jejunum versus ileum Small Intestine in Situ (B. Kathleen Alsup & Glenn M. Fox, University of Michigan Medical School, BlueLink) Schematic cross-section of small intestine Design & Artwork: The HIVE (hive.med.ubc.ca) 1 Midgut & Hindgut Organs & Their Blood Supply Lab 2 January 12, 2021 - Dr. Doroudi ([email protected]) Comparison of ileum and jejunum dissections Main features of ileum: thinner wall, no circular folds, many Peyer’s patches Design & Artwork: The HIVE (hive.med.ubc.ca) 2 Midgut & Hindgut Organs & Their Blood Supply Lab 2 January 12, 2021 - Dr. Doroudi ([email protected]) Viscera: Large intestine Appendix Ascending, transverse, descending, sigmoid portions of colon Rectum and anal canal (will be examined with pelvis) Taeniae coli, haustra coli, epiploic (omental) coli Large Intestine in Situ (B. -

Anatomy of the Small Intestine
Anatomy of the small intestine Make sure you check this Correction File before going through the content Small intestine Fixed Free (Retro peritoneal part) (Movable part) (No mesentery) (With mesentery) Duodenum Jejunum & ileum Duodenum Shape C-shaped loop Duodenal parts Length 10 inches Length Level Beginning At pyloro-duodenal Part junction FIRST PART 2 INCHES L1 (Superior) (Transpyloric Termination At duodeno-jejunal flexure Plane) SECOND PART 3 INCHES DESCENDS Peritoneal Retroperitoneal (Descending FROM L1 TO covering L3 Divisions 4 parts THIRD PART 4 INCHES L3 (SUBCOTAL (Horizontal) PLANE) Embryologic Foregut & midgut al origin FOURTH PART 1 INCHES ASCENDS Lymphatic Celiac & superior (Ascending) FROM L3 TO drainage mesenteric L2 Arterial Celiac & superior supply mesenteric Venous Superior mesenteric& Drainage Portal veins Duodenal relations part Anterior Posterior Medial Lateral First part Liver Bile duct - - Gastroduodenal artery Portal vein Second Part Liver Transverse Right kidney Pancreas Right colic flexure Colon Small intestine Third Part Small intestine Right psoas major - - Superior Inferior vena cava mesenteric vessels Abdominal aorta Inferior mesenteric vessels Fourth Part Small intestine Left psoas major - - Openings in second part of the duodenum Opening of accessory Common opening of bile duct pancreatic duct (one inch & main pancreatic duct: higher): on summit of major duodenal on summit of minor duodenal papilla. papilla. Jejunum & ileum Shape Coiled tube Length 6 meters (20 feet) Beginning At duodeno-jejunal flexur -

Histogenesis of the Proventricular Submucosal Gland of the Chick As Revealed by Light and Electron Microscopy Dale S
HISTOGENESIS OF THE PROVENTRICULAR SUBMUCOSAL GLAND OF THE CHICK AS REVEALED BY LIGHT AND ELECTRON MICROSCOPY DALE S. THOMSON Malone College, Canton, Ohio ABSTRACT The development of the submucosal tubuloalveolar glands of the proventriculus was studied with both light and electron microscopes, with special reference given to the 13-18-day period. Beginning as an invagination in the stratified columnar epithelium lining the lumen of the proventriculus, the simple tubular gland, by repeated bifurcation of the invaginated portion, by sloughing of the superficial cells, and by remodeling of the residual basal cells from columnar to cuboidal, becomes a compound tubuloalveolar gland composed of numerous secretory units. Concomitant with the gross cellular changes, ultra- structural changes in the intercellular membranes are evident. By day 4 after hatching, the cells forming the secretory units appear to be functional. INTRODUCTION The avian stomach is peculiar in that it consists of two morphologically and physiologically distinct parts: the glandular portion, or proventriculus, and the muscular portion, or gizzard. The proventriculus is a relatively thick-walled structure located at the distal end of the oesophagus and containing both simple mucous-secreting glands and compound submucosal tubuloalveolar glands. The gizzard is a thick muscular bulb, the mucosa of which contains simple glandular cells which secrete a tough, horny layer. The proventriculus has been described by Cazin (1886, 1887), Zietschmann (1908), Calhoun (1933, 1954), Batt (1924), and Bradley and Graham (1950). Aitken (1958) and Toner (1963) further amplified the literature with specific reference to the compound submucosal glands. Sjogren (1941) and Hibbard (1942) described the embryonic development of the submucosal glands.