Infectious Diseases
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

IDSA Guideline Review
1 Infectious Diseases Society of America Antimicrobial Resistant Treatment Guidance: Gram- Negative Bacterial Infections A Focus on Extended-Spectrum β-lactamase Producing Enterobacterales (ESBL-E), Carbapenem- Resistant Enterobacterales (CRE), and Pseudomonas aeruginosa with Difficult-to-Treat Resistance (DTR- P. aeruginosa) Guideline Summary by Kendra Suder, PharmD Candidate General Recommendations Considerations for choosing empiric therapy: o Local susceptibility patterns/ antibiogram o Patient’s previous organisms isolated & susceptibility data in the last 6 months o Antibiotic exposure in the last 30 days What if a patient was empirically placed on an antibiotic regimen that is now considered inactive against the organism based on susceptibility data? o If an inactive agent was started empirically for cystitis and patient improves, no change in antibiotic or extension of therapy is necessary o However, for other types of infections, guidelines recommend a change to an active regimen for a full treatment course (dated from the start of active therapy) Extended-Spectrum β-Lactamase-Producing Enterobacterales (ESBL-E) Extended-spectrum β-lactamase o Enzymes that inactivate most penicillins, cephalosporins, and aztreonam o Most commonly produced by Escherichia coli, Klebsiella pneumoniae, Klebsiella oxytoca, and Proteus mirabilis (guideline recommendations refer to ESBL-E infections caused by these organisms) o Most common type in the US is the CTX-M, especially CTX-M-15 In institutions that do not perform ESBL testing, a lack of -

Recarbrio, INN-Imipenem / Cilastatin / Relebactam
EMA/572792/2020 EMEA/H/C/004808 Recarbrio (imipenem / cilastatin / relebactam) An overview of Recarbrio and why it is authorised in the EU What is Recarbrio and what is it used for? Recarbrio is an antibiotic for treating adults with the following infections: • lung infections caught in hospital (hospital-acquired pneumonia), including ventilator-associated pneumonia (pneumonia caught while on a ventilator, which is a machine that helps a patient to breathe); • infection that has spread into the blood (bacteraemia) as a likely complication of hospital-acquired pneumonia or ventilator-associated pneumonia; • infections caused by bacteria classed as aerobic Gram-negative bacteria when other treatments might not work. Official guidance on the appropriate use of antibiotics should be considered when using the medicine. Recarbrio contains the active substances imipenem, cilastatin and relebactam. How is Recarbrio used? Recarbrio can only be obtained with a prescription and it should be used only after consulting a doctor with experience of managing infectious diseases. Recarbrio is given by infusion (drip) into a vein over 30 minutes. It is given every 6 hours for 5 to 14 days, depending on the nature of the infection. For more information about using Recarbrio, see the package leaflet or contact your doctor or pharmacist. How does Recarbrio work? One of the active substances in Recarbrio, imipenem, kills bacteria and the other two, cilastatin and relebactam, increase imipenem’s effectiveness in different ways. Imipenem interferes with bacterial proteins that are important for building the bacterial cell wall. This results in defective cell walls that collapse and cause the bacteria to die. -

New Β-Lactamase Inhibitor Combinations: Options for Treatment; Challenges for Testing
MEDICAL/SCIENTIFIC AffAIRS BULLETIN New β-lactamase Inhibitor Combinations: Options for Treatment; Challenges for Testing Background The β-lactam class of antimicrobial agents has played a crucial role in the treatment of infectious diseases since the discovery of penicillin, but β–lactamases (enzymes produced by the bacteria that can hydrolyze the β-lactam core of the antibiotic) have provided an ever expanding threat to their successful use. Over a thousand β-lactamases have been described. They can be divided into classes based on their molecular structure (Classes A, B, C and D) or their function (e.g., penicillinase, oxacillinase, extended-spectrum activity, or carbapenemase activity).1 While the first approach to addressing the problem ofβ -lactamases was to develop β-lactamase stable β-lactam antibiotics, such as extended-spectrum cephalosporins, another strategy that has emerged is to combine existing β-lactam antibiotics with β-lactamase inhibitors. Key β-lactam/β-lactamase inhibitor combinations that have been used widely for over a decade include amoxicillin/clavulanic acid, ampicillin/sulbactam, and pipercillin/tazobactam. The continued use of β-lactams has been threatened by the emergence and spread of extended-spectrum β-lactamases (ESBLs) and more recently by carbapenemases. The global spread of carbapenemase-producing organisms (CPOs) including Enterobacteriaceae, Pseudomonas aeruginosa, and Acinetobacter baumannii, limits the use of all β-lactam agents, including extended-spectrum cephalosporins (e.g., cefotaxime, ceftriaxone, and ceftazidime) and the carbapenems (doripenem, ertapenem, imipenem, and meropenem). This has led to international concern and calls to action, including encouraging the development of new antimicrobial agents, enhancing infection prevention, and strengthening surveillance systems. -

AMEG Categorisation of Antibiotics
12 December 2019 EMA/CVMP/CHMP/682198/2017 Committee for Medicinal Products for Veterinary use (CVMP) Committee for Medicinal Products for Human Use (CHMP) Categorisation of antibiotics in the European Union Answer to the request from the European Commission for updating the scientific advice on the impact on public health and animal health of the use of antibiotics in animals Agreed by the Antimicrobial Advice ad hoc Expert Group (AMEG) 29 October 2018 Adopted by the CVMP for release for consultation 24 January 2019 Adopted by the CHMP for release for consultation 31 January 2019 Start of public consultation 5 February 2019 End of consultation (deadline for comments) 30 April 2019 Agreed by the Antimicrobial Advice ad hoc Expert Group (AMEG) 19 November 2019 Adopted by the CVMP 5 December 2019 Adopted by the CHMP 12 December 2019 Official address Domenico Scarlattilaan 6 ● 1083 HS Amsterdam ● The Netherlands Address for visits and deliveries Refer to www.ema.europa.eu/how-to-find-us Send us a question Go to www.ema.europa.eu/contact Telephone +31 (0)88 781 6000 An agency of the European Union © European Medicines Agency, 2020. Reproduction is authorised provided the source is acknowledged. Categorisation of antibiotics in the European Union Table of Contents 1. Summary assessment and recommendations .......................................... 3 2. Introduction ............................................................................................ 7 2.1. Background ........................................................................................................ -

University of Pittsburg Medical Center Acquisition of Western Maryland
Andrew N. Pollak, M.D. Ben Steffen CHAIR EXECUTIVE DIRECTOR MARYLAND HEALTH CARE COMMISSION 4160 PATTERSON AVENUE – BALTIMORE, MARYLAND 21215 TELEPHONE: 410-764-3460 FAX: 410-358-1236 January 29, 2020 Via E-Mail and USPS Howard L. Sollins, Esquire Baker Donelson 100 Light Street Baltimore, Maryland 21202 Re: Acquisition of Health Care Facilities: (1) Western Maryland Regional Medical Center 12500 Willowbrook Road Cumberland, Maryland 21502 (2) Western Maryland Health System Frostburg Nursing and Rehabilitation Center 48 Tarn Terrace Frostburg, Maryland 21532 (3) Western Maryland Health System Home Care 1050 West Industrial Boulevard, Suite 19 Cumberland, Maryland 21502 (4) Western Maryland Health System Hospice Services 1050 West Industrial Boulevard, Suite 19 Cumberland, Maryland 21502 Dear Mr. Sollins: I write in response to your letter of December 16, 2019, notifying the Maryland Health Care Commission (MHCC) of the intent of University of Pittsburgh Medical Center (UPMC) to become the sole member of Western Maryland Health System (WMHS) through an integration and affiliation agreement. This acquisition of WMHS will constitute the acquisition of four Maryland “health care facilities,” as that term is defined in Maryland Certificate of Need Law. Those facilities are: 1. Western Maryland Regional Medical Center, a general hospital, which operates a special rehabilitation hospital on its campus; 2. Western Maryland Health System Frostburg Nursing and Rehabilitation Center (FNRC), a comprehensive care facility or nursing home; TDD FOR DISABLED TOLL FREE MARYLAND RELAY SERVICE 1-877-245-1762 1-800-735-2258 Howard L. Sollins, Esquire January 29, 2020 Page 2 3. Western Maryland Health System Home Care, a home health agency, authorized to serve patients in Allegany and Garrett Counties; and 4. -

Highmark/Upmc Agreement Provider Q&A
HIGHMARK/UPMC AGREEMENT PROVIDER Q&A JULY 2019 Highmark and UPMC have agreed to a 10-year contract offering full in-network access for Highmark members in certain health products that include UPMC providers and facilities in their networks in the Pittsburgh and Erie areas. Below are some questions and answers about this agreement. Q. What does this agreement mean to community hospitals? A. Highmark Health is committed to its strategy of community-based, close-to-home care for its members and patients. Community hospitals will continue to play an important role in serving the needs of our members and patients going forward. A contract with UPMC does not change that. Q. What does this agreement mean for your patients right now? A. Commercial (non-ACA, non-MA) • In Western PA, commercial members in high-performing narrow network products such as Community Blue Flex or Connect Blue are in a high-quality, lower-cost plan. Members in these products will have access to some, but not all, UPMC facilities and doctors effective July 1, 2019. This is very similar to what they had before the negotiation of this new agreement. • Certain UPMC Providers will be in-network at the highest tier. These include: o UPMC Altoona o UPMC Bedford o UPMC Cole o UPMC Horizon (for Community Blue Flex, Horizon is moving from Standard to Enhanced tier) o UPMC Jameson (for Community Blue Flex, Jameson is moving from Standard to Enhanced tier) o UPMC Kane o UPMC Northwest o UPMC Somerset o Western Psychiatric Institute and Clinic of UPMC o UPMC physicians and ancillary providers affiliated with the hospitals listed above o Over 20 UPMC and Community Hospital Cancer Centers (e.g., Excela Arnold Palmer Cancer Center, St. -

Title. 1 in Vitro Activity of the New Β-Lactamase Inhibitors Relebactam
bioRxiv preprint doi: https://doi.org/10.1101/499830; this version posted December 19, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Title. 2 In vitro activity of the new β-lactamase inhibitors relebactam and vaborbactam in combination 3 with β-lactams against Mycobacterium abscessus complex clinical isolates 4 5 Authors and affiliations. Amit Kaushik,a Nicole C. Ammerman,a Jin Lee,a Olumide Martins,a 6 Barry N. Kreiswirth,b Gyanu Lamichhane,a Nicole M. Parrish,c Eric L. Nuermbergera 7 8 aCenter for Tuberculosis Research, Johns Hopkins University School of Medicine, Baltimore, 9 Maryland, USA 10 bPublic Health Research Institute Tuberculosis Center, New Jersey Medical School - Rutgers, 11 The State University of New Jersey, Newark, New Jersey, USA 12 cDepartment of Pathology, Johns Hopkins University School of Medicine, Baltimore, Maryland, 13 USA 14 15 Key words. β-lactamase inhibitors, β-lactams, relebactam, vaborbactam, carbapenems, 16 cephalosporins, Mycobacterium abscessus 17 18 Running title. Relebactam/vaborbactam with β-lactams vs. M. abscessus Page 1 bioRxiv preprint doi: https://doi.org/10.1101/499830; this version posted December 19, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 19 Abstract 20 Pulmonary disease due to infection with Mycobacterium abscessus complex (MABC) is 21 notoriously difficult to treat, in large part due to MABC’s intrinsic resistance to most antibiotics, 22 including β-lactams. -

UPMC Standard Network
UPMC Standard Network UPMC Health Plan participating hospitals for Effective February 2020 commercial employer group plans In-network hospitals and facilities Allegheny Bradford Elk Curahealth Pittsburgh Guthrie Towanda Memorial Penn Highlands Elk Heritage Valley Health System – Hospital Erie Heritage Valley Sewickley Robert Packer Hospital Corry Memorial Hospital LifeCare Behavioral Health Hospital of Troy Community Hospital Millcreek Community Hospital Pittsburgh Butler Select Specialty Hospital – Erie LifeCare Hospitals of Pittsburgh at Butler Memorial Hospital UPMC Hamot Main Campus UPMC Passavant – Cranberry Ohio Valley Hospital Fayette Select Specialty Hospital – McKeesport Cambria Highlands Hospital Select Specialty Hospital – Conemaugh Memorial Medical Center Uniontown Hospital Conemaugh Miners Medical Center Pittsburgh UPMC Greene St. Clair Hospital Select Specialty Hospital – Johnstown Washington Health System Greene The Children’s Home of Pittsburgh Chester The Children’s Institute of Pittsburgh Huntingdon Bryn Mawr Rehab Hospital Penn Highlands Huntingdon UPMC Children’s Hospital of Pittsburgh Paoli Hospital UPMC East Indiana UPMC Hillman Cancer Center Clarion Indiana Regional Medical Center Clarion Hospital UPMC Magee-Womens Hospital Jefferson UPMC McKeesport Clearfield Penn Highlands Brookville UPMC Mercy Penn Highlands Clearfield Punxsutawney Area Hospital UPMC Montefiore Penn Highlands DuBois Lancaster UPMC Passavant – McCandless Clinton UPMC Lititz UPMC Presbyterian UPMC Lock Haven UPMC St. Margaret Lawrence UPMC Shadyside Crawford UPMC Jameson UPMC Western Psychiatric Hospital Meadville Medical Center Lycoming Titusville Area Hospital Armstrong UPMC Muncy Armstrong County Memorial Hospital Cumberland UPMC Williamsport UPMC Carlisle UPMC Williamsport Divine Providence Beaver UPMC Pinnacle West Shore Campus Curahealth Hospital Heritage Valley Dauphin McKean Heritage Valley Health System – Penn State Health Children’s Hospital Bradford Regional Medical Center Heritage Valley Beaver Penn State Health Milton S. -

Antibiotics Currently in Clinical Development
A data table from Sept 2014 Antibiotics Currently in Clinical Development As of September 2014, an estimated 38 new antibiotics1 with the potential to treat serious bacterial infections are in clinical development for the U.S. market. The success rate for drug development is low; at best, only 1 in 5 candidates that enter human testing will be approved for patients.* This snapshot of the antibiotic pipeline will be updated periodically as products advance or are known to drop out of development. Please contact Rachel Zetts at [email protected] or 202-540-6557 with additions or updates. Cited for potential Development Known QIDP4 Drug name Company Drug class activity against gram- Potential indication(s)?5 phase2 designation? negative pathogens?3 Approved (for acute Acute bacterial skin and skin structure bacterial skin and skin infections, hospital-acquired bacterial Tedizolid Cubist Pharmaceuticals Oxazolidinone Yes structure infections), pneumonia/ventilator-acquired bacterial June 20, 2014 pneumonia Approved, May 23, Acute bacterial skin and skin structure Dalbavancin Durata Therapeutics Lipoglycopeptide Yes 2014 infections Approved, August 6, Acute bacterial skin and skin structure Oritavancin The Medicines Company Glycopeptide Yes 2014 infections New Drug Application (NDA) submitted (for Complicated urinary tract infections, complicated urinary complicated intra-abdominal infections, Novel cephalosporin+beta- Ceftolozane+tazobactam8 tract infection and Cubist Pharmaceuticals Yes Yes acute pyelonephritis (kidney infection), lactamase -

CLSI AST News Update Janet A
Volume 3, Issue 2 Spring 2018 CLSI Subcommittee on Antimicrobial Susceptibility Testing CLSI AST News Update Janet A. Hindler, MCLS MT(ASCP) F(AAM), Editor Audrey N. Schuetz, MD, MPH, D(ABMM), Editor The CLSI Outreach Working Group (ORWG) is providing this Newsletter to highlight some recent issues related to antimicrobial susceptibility testing Inside This Issue: and reporting. We are listing links to some new educational materials and reminding you where you can find information about the CLSI AST Featured Article: Part 1 Subcommittee proceedings. New β-lactam combination agents for the treatment of Gram-negative bacterial infections: what the clinical microbiologist needs to know! ..................................................4 Upcoming Webinar: Featured Article: Part 2 Why all the fuss over quality control of Preparation, Presentation, and Promotion of Cumulative Antibiograms To β-lactam combination agents? ........................8 Support Antimicrobial Stewardship Programs Case Study: Cefazolin, Urine, and October 16, 2018 | 1:00–2:00 PM Eastern (US) Time Escherichia coli, Klebsiella pneumoniae, and Presenters: Proteus mirabilis: Entertaining Solutions Sharon Erdman, PharmD for Antimicrobial Susceptibility Testing and Clinical Professor, Purdue University College of Pharmacy Reporting ..........................................................12 Infectious Diseases Clinical Pharmacist/Co-Director OPAT Program, Eskenazi Health Burning Question: What Should Clinical Laboratorians Know About Gonorrhea in Patricia J. Simner, PhD, D(ABMM) 2018? ................................................................15 Associate Professor of Pathology, Johns Hopkins University Director of Medical Bacteriology and Parasitology Laboratories, Johns Hopkins Hot Topic: It’s Enough to mec You Crazy! ...18 Hospital What does the CLSI AST Subcommittee do? The first edition of the CLSI AST News Update (Vol 1, Issue 1, Spring 2016) described details about the organization and operation of the CLSI AST Subcommittee. -
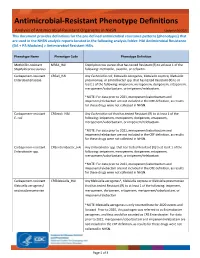
Antimicrobial Resistant Phenotype Definitions
Antimicrobial-Resistant Phenotype Definitions Analysis of Antimicrobial-Resistant Organisms in NHSN Updated 06/2021 This document provides definitions for the pre-defined antimicrobial resistance patterns (phenotypes) that are used in the NHSN analytic reports located in the following analysis folder: HAI Antimicrobial Resistance (DA + PA Modules) > Antimicrobial Resistant HAIs. Phenotype Name Phenotype Code Phenotype Definition Methicillin-resistant MRSA_HAI Staphylococcus aureus that has tested Resistant (R) to at least 1 of the Staphylococcus aureus following: methicillin, oxacillin, or cefoxitin. Carbapenem-resistant CREall_HAI Any Escherichia coli, Klebsiella aerogenes, Klebsiella oxytoca, Klebsiella Enterobacteriaceae pneumoniae, or Enterobacter spp. that has tested Resistant (R) to at least 1 of the following: imipenem, meropenem, doripenem, ertapenem, meropenem/vaborbactam, or imipenem/relebactam. *NOTE: For data prior to 2021, meropenem/vaborbactam and imipenem/relebactam are not included in the CRE definition, as results for these drugs were not collected in NHSN. Carbapenem-resistant CREecoli_HAI Any Escherichia coli that has tested Resistant (R) to at least 1 of the E. coli following: imipenem, meropenem, doripenem, ertapenem, meropenem/vaborbactam, or imipenem/relebactam. *NOTE: For data prior to 2021, meropenem/vaborbactam and imipenem/relebactam are not included in the CRE definition, as results for these drugs were not collected in NHSN. Carbapenem-resistant CREenterobacter_HAI Any Enterobacter spp. that has tested Resistant -

Acute-Care-Hospitals-Alphabetic.Pdf
Acute Care Hospitals Facility Name Facility # Facility Name Facility # Abington Health Center - Warminster 1410-09 Barnabas Health Jersey City Medical Center 2312-70 Campus(Warminster Hospital) Barnes-Kasson Hospital 1241-58 Abington Health-Lansdale Hospital 1432-46 Barnesville Hospital - Barnesville 1012-72 Abington Memorial Hospital 1001-46 Bath VA Medical Center 1062-71 Acuity Specialty Hospital of New Jersey (LTAC) 2310-70 (within Atlanticare Reg. Med.Ctr. Atlantic City Bayhealth Hospital, Sussex Campus 1012-68 Campu Bayshore Community Hospital 1039-70 AHN Harmar Neighborhood Hospital 1465-02 Beebe Medical Center - Lewes, DE 1003-68 AHN Hempfield Neighborhood Hospital 1130-65 Bellevue Hospital Center- New York, NY 1012-71 AHN McCandless Neighborhood Hospital 1464-02 Belmont Community Hospital (The Bellaire City 1002-72 AHN Neighborhood Hospital - Brentwood 1463-02 Hospital) Akron General Medical Center 1033-72 Belmont Hospital Bel Air 1026-72 Albany Medical Center Hospital - Albany, NY 1043-71 Benedictine Hospital 1001-71 Aliquippa Community Hospital (UPMC Beaver 1002-04 Berwick Hospital Center 1013-19 Valley Hospital) Beth Israel Hospital - Newark 1035-70 Allegheny General Hospital 1184-02 Beth Israel Med Ctr-Petrie Division (Manhattan) 1057-71 Allegheny Valley Hospital (Alle-Kiski Medical 1124-02 Center) Bloomsburg Hospital 1016-19 Anne Arundel Medical Center 1035-69 Bluefield Regional Medical Center 1029-73 Ardern Hill Hospital - Goshen 1017-71 Blythedale Children's Hospital 1063-71 Aria Health - Bucks County (formerly Delaware