Common Neuropathic Itch Syndromes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Notalgia Paresthetica: Cervical Spine Disease and Neuropathic Pruritus
Open Access Case Report DOI: 10.7759/cureus.12975 Notalgia Paresthetica: Cervical Spine Disease and Neuropathic Pruritus Ayesha Akram 1 1. Internal Medicine, Rawalpindi Medical University, Rawalpindi, PAK Corresponding author: Ayesha Akram, [email protected] Abstract Notalgia paresthetica (NP) is a dermatologic condition with predominant, primarily left unilateral pruritus and hyperpigmentation that typically occurs on the upper and middle back. The etiology remains largely elusive. A 57-year-old female with a history of neck pain presented with refractory NP since six months. Through diagnostic x-ray, cervical degenerative changes were discovered at the C5-C6 level, and she was prescribed a course of cervical traction. The cervical theory of NP is presented and is supported with x-ray findings in this case. Categories: Dermatology, Neurology Keywords: notalgia paresthetica, cervical spondylosis, enigmatic link Introduction Notalgia paresthetica (NP) is a cutaneous sensory neuropathy that predominantly affects females, with onset at middle age or older [1,2]. Although it is common, patients underestimate their symptoms, and physicians present an inertia to consider the possibility of NP, and far fewer know about the neuropathic itch. Doubtless, many cases go largely unrecognized, underdiagnosed, or overlooked in the routine clinical practice [3,4]. Pruritus is the overwhelming clinical symptom in the majority of patients [1,5]. A left-sided and posterior location matches well with the location of NP; almost always, NP is unilateral [1]. Hyperpigmentation in the affected area often results from scratching itchy, desensate skin [1]. Along with the pruritus, patients may also experience burning, tingling, coldness, hyperesthesia, hypoesthesia, numbness, or nerve pain in the area where pruritus appeared [6]. -
A Case Report of Refractory Notalgia Paresthetica Treated with Lidocaine
ISSN 1941-5923 © Am J Case Rep, 2017; 18: 1225-1228 DOI: 10.12659/AJCR.905676 Received: 2017.06.07 Accepted: 2017.06.28 A Case Report of Refractory Notalgia Published: 2017.11.20 Paresthetica Treated with Lidocaine Infusions Authors’ Contribution: BEF 1 Yaroslava Chtompel 1 Department of Anesthesiology and Chronic Pain Management, University Study Design A ABE 2 Marzieh Eghtesadi Hospital of Montreal (CHUM), Montreal, QC, Canada Data Collection B 2 Department of Chronic Pain and Headache Medicine, University Hospital of Statistical Analysis C ADE 1 Grisell Vargas-Schaffer Montreal (CHUM), Montreal, QC, Canada Data Interpretation D Manuscript Preparation E Literature Search F Funds Collection G Corresponding Author: Yaroslava Chtompel, e-mail: [email protected] Conflict of interest: None declared Patient: Female, 50 Final Diagnosis: Notalgia parethetica Symptoms: Hyperalgesia • Pruritus Medication: — Clinical Procedure: Intravenous lidocaine infusion Specialty: Anesthesiology Objective: Unusual or unexpected effect of treatment Background: Notalgia paresthetica is a neuropathic condition that manifests as a chronic itch in the thoraco-dorsal region. It is often resistant to treatment, and specific guidelines for its management are lacking. As such, we present a treatment approach with intravenous lidocaine infusions. Case Report: The case involves a 50-year-old woman with spinal cord injury caused by an epidural abscess. The patient de- veloped notalgia paresthetica and sublesional neuropathic pain following its drainage. -

Herpes Zoster by Lesia Dropulic, MD
Herpes Zoster Lesia Dropulic Jeffrey Cohen Laboratory of Infectious Diseases, NIAID Varicella (Chickenpox) Centers for Disease Control and Prevention Zoster (Shingles) Centers for Disease Control and Prevention Zoster is Due to Reactivation of Varicella from the Nervous System Blood Adapted from Kimberlin and Whitley NEJM 2007 VZV DNA is Present In Neurons in Ganglia Years After Chickenpox Ganglia latently infected with VZV Subject No. Neurons No. (%) neurons Median VZV DNA Number Tested positive for VZV copies/positive cell Wang et al J Virol 2005 History of Zoster • Zoster: Greek for girdle • Shingles: Latin (cingere) girdle Partial encircling of the trunk with rash First Cell Culture of Varicella-Zoster Virus (March 19, 1949) Thomas Weller in Varicella-Zoster Virus, Cambridge Press 2000 Varicella- Zoster Virus Straus et al. Ann Intern Med 1988 Epidemiology of Zoster • About 99% of adults >40 yo infected with varicella-zoster, thus all older adults at risk • About 1 million cases in the US each year • Rates appear to be increasing • 50% of persons of live to age 85 will develop zoster, 5% may get a second case Risk Factors for Zoster • Age- the major risk factor for healthy persons (long duration since exposure to virus) • Immune compromise- T cell immunity: transplant recipients, leukemia, lymphoma; HIV increases the risk up to 50 fold • Age and immune compromise- reduced VZV-specific T cell immunity Varicella-Zoster Virus: Site of Latency • Varicella-zoster virus is latent in dorsal root ganglia (along the spine) or cranial nerve -

Therapeutic Use of Botulinum Neurotoxins in Dermatology: Systematic Review
toxins Review Therapeutic Use of Botulinum Neurotoxins in Dermatology: Systematic Review Emanuela Martina †, Federico Diotallevi †, Giulia Radi †, Anna Campanati * and Annamaria Offidani Dermatological Clinic, Department of Clinical and Molecular Sciences, Polytechnic Marche University, 60020 Ancona, Italy; [email protected] (E.M.); [email protected] (F.D.); [email protected] (G.R.); annamaria.offi[email protected] (A.O.) * Correspondence: [email protected] † These authors equally contributed to the manuscript. Abstract: Botulinum toxin is a superfamily of neurotoxins produced by the bacterium Clostridium Botulinum with well-established efficacy and safety profile in focal idiopathic hyperhidrosis. Recently, botulinum toxins have also been used in many other skin diseases, in off label regimen. The objective of this manuscript is to review and analyze the main therapeutic applications of botulinum toxins in skin diseases. A systematic review of the published data was conducted, following Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines. Botulinum toxins present several label and off-label indications of interest for dermatologists. The best-reported evidence concerns focal idiopathic hyperhidrosis, Raynaud phenomenon, suppurative hidradenitis, Hailey–Hailey disease, epidermolysis bullosa simplex Weber–Cockayne type, Darier’s disease, pachyonychia congenita, aquagenic keratoderma, alopecia, psoriasis, notalgia paresthetica, facial erythema and flushing, and oily skin. -

The Neuroanatomy of Female Pelvic Pain
Chapter 2 The Neuroanatomy of Female Pelvic Pain Frank H. Willard and Mark D. Schuenke Introduction The female pelvis is innervated through primary afferent fi bers that course in nerves related to both the somatic and autonomic nervous systems. The somatic pelvis includes the bony pelvis, its ligaments, and its surrounding skeletal muscle of the urogenital and anal triangles, whereas the visceral pelvis includes the endopelvic fascial lining of the levator ani and the organ systems that it surrounds such as the rectum, reproductive organs, and urinary bladder. Uncovering the origin of pelvic pain patterns created by the convergence of these two separate primary afferent fi ber systems – somatic and visceral – on common neuronal circuitry in the sacral and thoracolumbar spinal cord can be a very dif fi cult process. Diagnosing these blended somatovisceral pelvic pain patterns in the female is further complicated by the strong descending signals from the cerebrum and brainstem to the dorsal horn neurons that can signi fi cantly modulate the perception of pain. These descending systems are themselves signi fi cantly in fl uenced by both the physiological (such as hormonal) and psychological (such as emotional) states of the individual further distorting the intensity, quality, and localization of pain from the pelvis. The interpretation of pelvic pain patterns requires a sound knowledge of the innervation of somatic and visceral pelvic structures coupled with an understand- ing of the interactions occurring in the dorsal horn of the lower spinal cord as well as in the brainstem and forebrain. This review will examine the somatic and vis- ceral innervation of the major structures and organ systems in and around the female pelvis. -

Notalgia Paresthetica: Successful Treatment with Exercises
356 Letters to the Editor Notalgia Paresthetica: Successful Treatment with Exercises Anne B. Fleischer1, Tammy J. Meade1 and Alan B. Fleischer2* Departments of 1Physical and Occupational Therapy and 2Dermatology, Wake Forest University Health Sciences, 131 Miller Street, Winston-Salem, NC 27103, USA. *E-mail: [email protected] Accepted September 29, 2010. Notalgia paresthetica (NP) presents typically as a uni- Considering this anatomy, ABF noted that she sat lateral localized itch in the midscapular area. Although with rounded shoulders, which protracted and elevated the pathogenesis of NP has not been fully elucidated, it her scapulae and flexed her head and spine. Within this is widely believed to be a neurogenic itch resulting from position, the cutaneous spinal nerves are under constant spinal nerve impingement or chronic nerve trauma (1, stretch, which causes the spinal nerve angles to become 2). Massey & Fleet (3) postulate that spinal nerves from more severe. T2 to T6 emerge through the multifidus spinae muscle at Knowing that the nerves first pierce the rhomboid right angles and are therefore exposed to chronic trauma. and trapezius muscles prior to becoming cutaneous Further evidence supporting this neurologic causation nerves, ABF thought that she may be able to lessen resides in the reported effective treatment options, which this nerve angle if she strengthened her rhomboids include typical neuralgia therapies, such as topical capsai- and latissimus dorsi muscles as well as stretched the cin (4), gabapentin (5), oxcarbazepine (6), and botulinum pectoral muscles (Fig. 1). By completing these exer- toxin type A (7). In addition, Savk et al. (8) demonstrated cises and stretches, her posture changed from having the use of transcutaneous electrical nerve stimulation to reduce the symptoms of 15 adults with NP. -

Disc Pathology Lumbar Dermatomes
Lumbar Dermatomes: Disc Pathology Lumbar Dermatomes In this example, nerve root pain is due to disc pathology. Dermatomes are regions of altered sensation from irritated or damaged nerve roots. Symptoms that follow a dermatome (numbness, tingling or pain) may indicate a pathology that involves the related nerve root. These symptoms can follow the entire dermatome or just part of it. When symptoms cover more than one dermatome it may suggest more severe pathology and involvement of more than one nerve root. Disc Pathology: Lumbar Disc Pathology has various presentations. 1. Normal Disc 2. Internal Disc Disorder: Small tear at the inner part of the outer third of the disc where the annulus is innervated. 3. Outer Disc Disorder: Larger tear that extends to the outer part of the annulus. Discography is a good way to diagnose the morphology of the disc and establish the disc as a pain generator. 4. Protrusion: Small disc bulge and the outer layers of the annulus are intact 5. Prolapse: Large disc bulge that breaks through the layers of the annulus but not the posterior longitudinal ligament (PLL). 6. Extrusion: Large disc bulge that breaks through the layers of the annulus and the PLL. This often causes pain in a multitude of dermatomes. 7. Sequestration (not shown; rare): Disc fragment breaks away from the rest of the discs. 1. Sizer PS Jr, Phelps V, Matthijs O. Pain generators of the lumbar spine. Pain Pract. 2001;1(3):255‐ 273. 2. Sizer PS Jr, Phelps V, Dedrick G, Matthijs O. Differential diagnosis and management of spinal nerve root‐related pain. -

Unit #2 - Abdomen, Pelvis and Perineum
UNIT #2 - ABDOMEN, PELVIS AND PERINEUM 1 UNIT #2 - ABDOMEN, PELVIS AND PERINEUM Reading Gray’s Anatomy for Students (GAFS), Chapters 4-5 Gray’s Dissection Guide for Human Anatomy (GDGHA), Labs 10-17 Unit #2- Abdomen, Pelvis, and Perineum G08- Overview of the Abdomen and Anterior Abdominal Wall (Dr. Albertine) G09A- Peritoneum, GI System Overview and Foregut (Dr. Albertine) G09B- Arteries, Veins, and Lymphatics of the GI System (Dr. Albertine) G10A- Midgut and Hindgut (Dr. Albertine) G10B- Innervation of the GI Tract and Osteology of the Pelvis (Dr. Albertine) G11- Posterior Abdominal Wall (Dr. Albertine) G12- Gluteal Region, Perineum Related to the Ischioanal Fossa (Dr. Albertine) G13- Urogenital Triangle (Dr. Albertine) G14A- Female Reproductive System (Dr. Albertine) G14B- Male Reproductive System (Dr. Albertine) 2 G08: Overview of the Abdomen and Anterior Abdominal Wall (Dr. Albertine) At the end of this lecture, students should be able to master the following: 1) Overview a) Identify the functions of the anterior abdominal wall b) Describe the boundaries of the anterior abdominal wall 2) Surface Anatomy a) Locate and describe the following surface landmarks: xiphoid process, costal margin, 9th costal cartilage, iliac crest, pubic tubercle, umbilicus 3 3) Planes and Divisions a) Identify and describe the following planes of the abdomen: transpyloric, transumbilical, subcostal, transtu- bercular, and midclavicular b) Describe the 9 zones created by the subcostal, transtubercular, and midclavicular planes c) Describe the 4 quadrants created -

The Lymphatic Theory of Notalgia Paresthetica
FEATURE ARTICLE The Lymphatic Theory of Notalgia Paresthetica Carleen Willeford ABSTRACT: Notalgia paresthetica is a dermatologic con- of the clinical features. The distribution is adjacent to the dition with prominent primarily left unilateral pruritis and T2YT6 vertebrae, and degenerative changes of thoracic raised erythematous rash with hyperpigmentation at the spondylosis have been found in 60% and 75% of patients, medial or inferior scapula. The etiology is unknown. A which led to the conjecture that spinal nerve impingement comprehensive review of the literature was performed may contribute to the pathogenesis of NP (Raison-Peyron, with a structured analysis of previous theories. There is no Meunier, Acevedo, & Meynadier, 1999; Savk & Savk, consistent imaging or functional test to support any of 2005). This is based on the reasoning that there is nerve the previously proposed mechanisms. A new theory is compression from the spondylosis as the nerve roots exit the presented with a unifying theme of all previous treatments vertebral column at each level through the neural foramen. and is supported with results of the first electrical imped- However, this seems unreasonable because not all patients ance myography testing in this condition. have thoracic spondylosis; in those who do, the spondylosis Key words: Notalgia Paresthetica, Pruritis, Electrical is not limited to only the T2YT6 locations, and most Impedance Myography, Lymphatic Theory importantly, the location of NP is only medial, and not a dermatomal pattern, as would be expected from a neural otalgia paresthetica (NP) is a dermatologic foraminal impingement. Importantly, electrodiagnostic condition with prominent primarily left studies have not confirmed this. However, there was a unilateral pruritis and raised erythematous single report in the 1980s that identified electromyographic rash with hyperpigmentation at the medial paraspinal denervation at T2YT6 (Massey & Pleet, 1984). -

Dermatomal Distribution | Definition of Dermatomal Distribution by Medical
10/13/2016 Dermatomal distribution | definition of dermatomal distribution by Medical dictionary Dermatomal distribution | definition of dermatomal distribution by Medical dictionary http://medicaldictionary.thefreedictionary.com/dermatomal+distribution dermatome (redirected from dermatomal distribution) Also found in: Dictionary, Thesaurus, Encyclopedia, Wikipedia. dermatome [der´mah-tōm] 1. the area of skin supplied with afferent nerve fibers by a single posterior spinal root. 2. the lateral part of an embryonic somite. 3. an instrument for removing splitthickness skin grafts from donor sites; there are many different kinds, divided into three major types: knife, drum, and motordriven. Dermatomes. Segmental dermatome distribution of spinal nerves to the front, back, and side of the body. C, Cervical segments; T, thoracic segments; L, lumbar segments; S, sacral segments; CX, coccygeal segment. Dermatomes are specific skin surface areas innervated by a single spinal nerve or group of spinal nerves. Dermatome assessment is done to determine the level of spinal anesthesia for surgical procedures and postoperative analgesia when epidural local anesthetics are used. From Thibodeau and Patton, 1999. drum dermatome a dermatome consisting of a cylindrical drumlike apparatus coated with adhesive that rolls over the skin while a blade moves across the surface and cuts the graft free. knife dermatome the simplest type of dermatome, which is used to remove grafts by a freehand technique. motordriven dermatome a dermatome driven by a power source; motordriven dermatomes cut with a backandforth blade action. MillerKeane Encyclopedia and Dictionary of Medicine, Nursing, and Allied Health, Seventh Edition. © 2003 by Saunders, an imprint of Elsevier, Inc. -

Neuropathic Itch
cells Review Neuropathic Itch James Meixiong 1, Xinzhong Dong 2,3 and Hao-Jui Weng 4,5,* 1 Solomon H. Snyder Department of Neuroscience and Medical Scientist Training Program, Johns Hopkins University School of Medicine, Baltimore, MD 21205, USA; [email protected] 2 Solomon H. Snyder Department of Neuroscience, Department of Dermatology, and Department of Neurosurgery, Johns Hopkins University School of Medicine, Baltimore, MD 21205, USA; [email protected] 3 Howard Hughes Medical Institute, Johns Hopkins University School of Medicine, Baltimore, MD 21205, USA 4 Department of Dermatology, Taipei Medical University-Shuang Ho Hospital, New Taipei City 23561, Taiwan 5 Department of Dermatology, School of Medicine, College of Medicine, Taipei Medical University, Taipei 11031, Taiwan * Correspondence: [email protected] Received: 23 September 2020; Accepted: 8 October 2020; Published: 9 October 2020 Abstract: Neurologic insults as varied as inflammation, stroke, and fibromyalgia elicit neuropathic pain and itch. Noxious sensation results when aberrantly increased afferent signaling reaches percept-forming cortical neurons and can occur due to increased sensory signaling, decreased inhibitory signaling, or a combination of both processes. To treat these symptoms, detailed knowledge of sensory transmission, from innervated end organ to cortex, is required. Molecular, genetic, and behavioral dissection of itch in animals and patients has improved understanding of the receptors, cells, and circuits involved. In this review, we will discuss neuropathic itch with a focus on the itch-specific circuit. Keywords: pruritus; neuropathy; inflammation 1. Introduction Both microscopic damage, such as small-fiber neuropathy, and macroscopic trauma such as that occurring from spinal cord transection can result in neuropathic pain or itch [1]. -
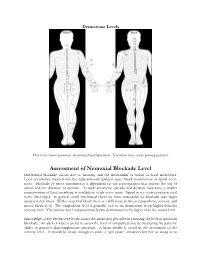
Assessment of Neuraxial Blockade Level Differential Blockade Occurs Due to Anatomy and the Mechanism of Action of Local Anesthetics
Dermatome Levels This is the most common anatomical configuration. Variation may occur among patients. Assessment of Neuraxial Blockade Level Differential blockade occurs due to anatomy and the mechanism of action of local anesthetics. Local anesthetics injected into the subarachnoid/epidural space block transmission at spinal nerve roots. Blockade of nerve transmission is dependent on the concentration that reaches the site of action and the duration of contact. As local anesthetic spreads and distance increases, a smaller concentration of local anesthetic is available to reach nerve roots. Spinal nerve roots contain several nerve fiber types. In general, small myelinated fibers are more susceptible to blockade than larger unmyelinated fibers. With a neuraxial block there is a difference between sympathetic, sensory, and motor block level. The sympathetic level is generally two to six dermatome levels higher than the sensory level. The sensory level is approximately two dermatome levels higher than the motor level. Knowledge of key dermatome levels assists the anesthesia provider in assessing the level of neuraxial blockade. An alcohol wipe is useful to assess the level of sympathectomy by measuring the patients’ ability to perceive skin temperature sensation. A blunt needle is useful in the assessment of the sensory level. It should be sharp enough to cause a “pin prick” sensation but not so sharp as to break the patients skin. The use of the spinal needle stylet can be used. Pinching the patient can also be used. The table below will help determine if the level of blockade achieves the minimum level required for a proposed surgical procedure. When reviewing the required sensory levels, it seems odd that the sensory level is higher than where the surgical procedure actually takes place.