The Evolution of the Mammalian Pharynx
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Reptile-Like Physiology in Early Jurassic Stem-Mammals
bioRxiv preprint doi: https://doi.org/10.1101/785360; this version posted October 10, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Title: Reptile-like physiology in Early Jurassic stem-mammals Authors: Elis Newham1*, Pamela G. Gill2,3*, Philippa Brewer3, Michael J. Benton2, Vincent Fernandez4,5, Neil J. Gostling6, David Haberthür7, Jukka Jernvall8, Tuomas Kankanpää9, Aki 5 Kallonen10, Charles Navarro2, Alexandra Pacureanu5, Berit Zeller-Plumhoff11, Kelly Richards12, Kate Robson-Brown13, Philipp Schneider14, Heikki Suhonen10, Paul Tafforeau5, Katherine Williams14, & Ian J. Corfe8*. Affiliations: 10 1School of Physiology, Pharmacology & Neuroscience, University of Bristol, Bristol, UK. 2School of Earth Sciences, University of Bristol, Bristol, UK. 3Earth Science Department, The Natural History Museum, London, UK. 4Core Research Laboratories, The Natural History Museum, London, UK. 5European Synchrotron Radiation Facility, Grenoble, France. 15 6School of Biological Sciences, University of Southampton, Southampton, UK. 7Institute of Anatomy, University of Bern, Bern, Switzerland. 8Institute of Biotechnology, University of Helsinki, Helsinki, Finland. 9Department of Agricultural Sciences, University of Helsinki, Helsinki, Finland. 10Department of Physics, University of Helsinki, Helsinki, Finland. 20 11Helmholtz-Zentrum Geesthacht, Zentrum für Material-und Küstenforschung GmbH Germany. 12Oxford University Museum of Natural History, Oxford, OX1 3PW, UK. 1 bioRxiv preprint doi: https://doi.org/10.1101/785360; this version posted October 10, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 13Department of Anthropology and Archaeology, University of Bristol, Bristol, UK. 14Faculty of Engineering and Physical Sciences, University of Southampton, Southampton, UK. -

Larynx Anatomy
LARYNX ANATOMY Elena Rizzo Riera R1 ORL HUSE INTRODUCTION v Odd and median organ v Infrahyoid region v Phonation, swallowing and breathing v Triangular pyramid v Postero- superior base àpharynx and hyoid bone v Bottom point àupper orifice of the trachea INTRODUCTION C4-C6 Tongue – trachea In women it is somewhat higher than in men. Male Female Length 44mm 36mm Transverse diameter 43mm 41mm Anteroposterior diameter 36mm 26mm SKELETAL STRUCTURE Framework: 11 cartilages linked by joints and fibroelastic structures 3 odd-and median cartilages: the thyroid, cricoid and epiglottis cartilages. 4 pair cartilages: corniculate cartilages of Santorini, the cuneiform cartilages of Wrisberg, the posterior sesamoid cartilages and arytenoid cartilages. Intrinsic and extrinsic muscles THYROID CARTILAGE Shield shaped cartilage Right and left vertical laminaà laryngeal prominence (Adam’s apple) M:90º F: 120º Children: intrathyroid cartilage THYROID CARTILAGE Outer surface à oblique line Inner surface Superior border à superior thyroid notch Inferior border à inferior thyroid notch Superior horns à lateral thyrohyoid ligaments Inferior horns à cricothyroid articulation THYROID CARTILAGE The oblique line gives attachement to the following muscles: ¡ Thyrohyoid muscle ¡ Sternothyroid muscle ¡ Inferior constrictor muscle Ligaments attached to the thyroid cartilage ¡ Thyroepiglottic lig ¡ Vestibular lig ¡ Vocal lig CRICOID CARTILAGE Complete signet ring Anterior arch and posterior lamina Ridge and depressions Cricothyroid articulation -
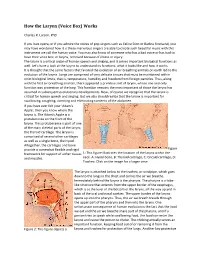
How the Larynx (Voice Box) Works
How the Larynx (Voice Box) Works Charles R. Larson, PhD If you love opera, or if you admire the voices of pop singers such as Celine Dion or Barbra Streisand, you may have wondered how it is these marvelous singers are able to create such beautiful music with this instrument we call the human voice. You may also know of someone who has a bad voice or has had to have their voice box, or larynx, removed because of illness or injury. The larynx is a critical organ of human speech and singing, and it serves important biological functions as well. Let's have a look at the larynx to understand its functions, what it looks like and how it works. It is thought that the same factors that favored the evolution of air‐breathing animals on earth led to the evolution of the larynx. Lungs are comprised of very delicate tissues that must be maintained within strict biological limits, that is, temperature, humidity and freedom from foreign particles. Thus, along with the first air‐breathing animals, there appeared a primitive sort of larynx, whose one and only function was protection of the lung. This function remains the most important of those the larynx has assumed in subsequent evolutionary developments. Now, of course we recognize that the larynx is critical for human speech and singing. But we also should realize that the larynx is important for swallowing, coughing, vomiting and eliminating contents of the abdomen. If you have ever felt your 'Adam's Apple', then you know where the larynx is. -

Understanding Your Child's Videofluoroscopy
Understanding your child’s videofluoroscopy swallow study report This leaflet will explain some of the words used by the speech and language therapist (SLT) in the letter sent out after the videofluoroscopy swallow study. The recommendations introduce some of the ways that your child’s problems with swallowing can be managed. If you have any questions or concerns, please speak to your SLT. What happens during swallowing? Swallowing is a series of movements that prepares food and fluid in the mouth, and then delivers it through the pharynx and oesophagus to the stomach. This is a diagram of the inside of the mouth and throat. You might find it useful to refer to when reading the information in this leaflet. 1 Tongue 2 Hard palate (roof of the mouth) 3 Soft palate (soft tissue at the back of the roof of the mouth) 4 Pharynx or throat (tube that connects the mouth and nostrils to the gullet) 5 Valeculae (depression below the root of the tongue) 6 Epiglottis (cartilage flap attached at the top of the larynx) 7 Pyriform sinuses (recesses on either side of the entrance to the larynx) 8 Larynx (the voice box, which is located at the top of the airway) 9 Vocal cords (two membranes which vibrate when speaking and move together when swallowing. This movement is a protective mechanism to stop food or drink entering the airway. The vocal cords are located in the voice box.) 10 Trachea (tube connecting the larynx to the lungs) 11 Upper oesophageal sphincter (muscular ring at the entrance to the oesophagus to reduce the risk of food coming back up) 12 Gullet or oesophagus (tube connecting the pharynx to the stomach) 1 of 3 Swallowing phases Swallowing involves three phases: 1. -

Larynx 2017‐2018 Naaccr Webinar Series
NAACCR 2017-2018 Webinar Series 11/2/2017 COLLECTING CANCER DATA: LARYNX 2017‐2018 NAACCR WEBINAR SERIES Q&A • Please submit all questions concerning webinar content through the Q&A panel. • Reminder: • If you have participants watching this webinar at your site, please collect their names and emails. • We will be distributing a Q&A document in about one week. This document will fully answer questions asked during the webinar and will contain any corrections that we may discover after the webinar. 2 Larynx 1 NAACCR 2017-2018 Webinar Series 11/2/2017 Fabulous Prizes 3 AGENDA • Anatomy • Epi Moment • Quiz 1 • Staging • Treatment • Quiz 2 • Case Scenarios 4 Larynx 2 NAACCR 2017-2018 Webinar Series 11/2/2017 ANATOMY LARYNX 5 LARYNX ANATOMY • Voice Box • Passageway of air • Extends from C3 to C6 vertebrae 6 Larynx 3 NAACCR 2017-2018 Webinar Series 11/2/2017 LARYNX ANATOMY • Divided into 3 Sections • Supraglottis • area above vocal cords, contains epiglottis • arytenoids, aryepiglottic folds and false cords • Glottis • containing true vocal cords, anterior and posterior commissures • Subglottis • below the vocal cords 7 LARYNX ANATOMY • Epiglottis • Aryepiglottic Folds • Anterior and Posterior • False vocal cords Commissure • True vocal cords • Arytenoids 8 Larynx 4 NAACCR 2017-2018 Webinar Series 11/2/2017 LARYNX ANATOMY • Thyroid cartilage • Arytenoid cartilage • Adam’s apple • Influence position and tension of the • Thyrohyoid membrane vocal cords • Cricoid cartilage • Corniculate cartilage • Inferior wall of larynx • Horn shaped pieces located -

Earthworm Dissection External Anatomy Examine Your Earthworm and Determine the Dorsal and Ventral Sides
Name(s): ___________________________________________Date:________ Earthworm Dissection External Anatomy Examine your earthworm and determine the dorsal and ventral sides. Locate the two openings on the ventral surface of the earthworm. Locate the openings toward the anterior of the worm which are the s perm ducts. Locate the openings near the clitellum which are the g enital setae . Locate the dark line that runs down the dorsal side of the worm, this is the dorsal blood vessel. The ventral blood vessel can be seen on the underside of the worm, though it is usually not as dark . Locate the worm’s anus on the far posterior end of the worm. Note the swelling of the earthworm near its anterior side - this is the clitellum. Label the earthworm pictured. A_____________________________ B _____________________________ C _____________________________ D _____________________________ Internal Anatomy 1. Place the specimen in the dissecting pan D ORSAL side up. 2. Locate the clitellum and insert the tip of the scissors about 3 cm posterior. 3. Cut carefully all the way up to the head. Try to keep the scissors pointed up, and only cut through the skin. 4. Spread the skin of the worm out, use a teasing needle to gently tear the s epta (little thread like structures that hold the skin to organs below it) 5. Place pins in the skin to hold it apart – set them at an angle so they aren’t in the way of your view. Reproductive System The first structures you probably see are the s eminal vesicles. They are cream colored and located toward the anterior of the worm. -

The Articulatory System Chapter 6 Speech Science/ COMD 6305 UTD/ Callier Center William F. Katz, Ph.D
The articulatory system Chapter 6 Speech Science/ COMD 6305 UTD/ Callier Center William F. Katz, Ph.D. STRUCTURE/FUNCTION VOCAL TRACT CLASSIFICATION OF CONSONANTS AND VOWELS MORE ON RESONANCE ACOUSTIC ANALYSIS/ SPECTROGRAMS SUPRSEGMENTALS, COARTICULATION 1 Midsagittal dissection From Kent, 1997 2 Oral Cavity 3 Oral Structures – continued • Moistened by saliva • Lined by mucosa • Saliva affected by meds 4 Tonsils • PALATINE* (laterally – seen in oral periph • LINGUAL (inf.- root of tongue) • ADENOIDS (sup.) [= pharyngeal] • Palatine, lingual tonsils are larger in children • *removed in tonsillectomy 5 Adenoid Facies • Enlargement from infection may cause problems (adenoid facies) • Can cause problems with nasal sounds or voicing • Adenoidectomy; also tonsillectomy (for palatine tonsils) 6 Adenoid faces (example) 7 Oral structures - frenulum Important component of oral periphery exam Lingual frenomy – for ankyloglossia “tongue-tie” Some doctors will snip for infants, but often will loosen by itself 8 Hard Palate Much variability in palate shape and height Very high vault 9 Teeth 10 Dentition - details Primary (deciduous, milk teeth) Secondary (permanent) n=20: n=32: ◦ 2 incisor ◦ 4 incisor ◦ 1 canine ◦ 2 canine ◦ 2 molar ◦ 4 premolar (bicuspid) Just for “fun” – baby ◦ 6 molar teeth pushing in! NOTE: x 2 for upper and lower 11 Types of malocclusion • Angle’s classification: • I, II, III • Also, individual teeth can be misaligned (e.g. labioversion) Also “Neutrocclusion/ distocclusion/mesiocclusion” 12 Dental Occlusion –continued Other terminology 13 Mandible Action • Primary movements are elevation and depression • Also…. protrusion/retraction • Lateral grinding motion 14 Muscles of Jaw Elevation Like alligators, we are much stronger at jaw elevation (closing to head) than depression 15 Jaw Muscles ELEVATORS DEPRESSORS •Temporalis ✓ •Mylohyoid ✓ •Masseter ✓ •Geniohyoid✓ •Internal (medial) Pterygoid ✓ •Anterior belly of the digastric (- Kent) •Masseter and IP part of “mandibular sling” •External (lateral) pterygoid(?)-- also protrudes and rocks side to side. -

Dr. Maue-Dickson Is Associate Professor of Pediat- Rics, University of Miami, Mailman Center for Child Development, University
Section II. Anatomy and Physiology WILMA MAUE-DICKSON, Ph.D. (CHAIRMAN) Introduction Middle Ear Musculature, The Auditory Tube, and The Velopharyngeal This Section has been prepared for the Mechanism purpose of updating the previous report, "Status of Research in Cleft Palate: Anat- 1. Tur Mippour® Ear omy and Physiology," published in two parts in the Cleft Palate Journal, Volume 11, The authors of the previous report 1974, and Volume 12, 1975. questioned the validity of the concept that As indicated in the previous two-part the tensor tympani and the stapedius mus- report, it is imperative to consider not only cles provide protection to the inner ear the palate but all of the oral-facial-pharyn- from loud sounds, except perhaps for geal system, both in normal and abnormal minimal protection (less than 10 dB) at low conditions, and both in the adult and in frequencies. They also cited research the developing child. Thus, this review in- which indicated that stapedius contraction cludes normal, abnormal, and develop- is more closely associated with voicing and mental studies on middle ear musculature, coughing than with acoustic stimuli, and the auditory tube, the velopharyngeal that the middle ear muscles might be in- mechanism, the tongue, the larynx, the volved in auditory tube opening. face and mandible, and blood supply and The literature reviewed for this report innervation relevant to cleft lip and palate. does not resolve all of these questions, but Though the relevance of embryology of it does add some focus for future research. the orofacial complex is obvious, it has Greisen and Neergaard (1975) used extra- been reviewed in a recently published re- tympanic phonometry to study middle ear port (Dickson, 1975) and will not be in- reflex activity and were able to demon- cluded as a separate topic in this review strate a tensor tympani reflex in response because of space limitations. -

Summary Report of Freshwater Nonindigenous Aquatic Species in U.S
Summary Report of Freshwater Nonindigenous Aquatic Species in U.S. Fish and Wildlife Service Region 4—An Update April 2013 Prepared by: Pam L. Fuller, Amy J. Benson, and Matthew J. Cannister U.S. Geological Survey Southeast Ecological Science Center Gainesville, Florida Prepared for: U.S. Fish and Wildlife Service Southeast Region Atlanta, Georgia Cover Photos: Silver Carp, Hypophthalmichthys molitrix – Auburn University Giant Applesnail, Pomacea maculata – David Knott Straightedge Crayfish, Procambarus hayi – U.S. Forest Service i Table of Contents Table of Contents ...................................................................................................................................... ii List of Figures ............................................................................................................................................ v List of Tables ............................................................................................................................................ vi INTRODUCTION ............................................................................................................................................. 1 Overview of Region 4 Introductions Since 2000 ....................................................................................... 1 Format of Species Accounts ...................................................................................................................... 2 Explanation of Maps ................................................................................................................................ -

The Myloglossus in a Human Cadaver Study: Common Or Uncommon Anatomical Structure? B
Folia Morphol. Vol. 76, No. 1, pp. 74–81 DOI: 10.5603/FM.a2016.0044 O R I G I N A L A R T I C L E Copyright © 2017 Via Medica ISSN 0015–5659 www.fm.viamedica.pl The myloglossus in a human cadaver study: common or uncommon anatomical structure? B. Buffoli*, M. Ferrari*, F. Belotti, D. Lancini, M.A. Cocchi, M. Labanca, M. Tschabitscher, R. Rezzani, L.F. Rodella Section of Anatomy and Physiopathology, Department of Clinical and Experimental Sciences, University of Brescia, Brescia, Italy [Received: 1 June 2016; Accepted: 18 July 2016] Background: Additional extrinsic muscles of the tongue are reported in literature and one of them is the myloglossus muscle (MGM). Since MGM is nowadays considered as anatomical variant, the aim of this study is to clarify some open questions by evaluating and describing the myloglossal anatomy (including both MGM and its ligamentous counterpart) during human cadaver dissections. Materials and methods: Twenty-one regions (including masticator space, sublin- gual space and adjacent areas) were dissected and the presence and appearance of myloglossus were considered, together with its proximal and distal insertions, vascularisation and innervation. Results: The myloglossus was present in 61.9% of cases with muscular, ligamen- tous or mixed appearance and either bony or muscular insertion. Facial artery pro- vided myloglossal vascularisation in the 84.62% and lingual artery in the 15.38%; innervation was granted by the trigeminal system (buccal nerve and mylohyoid nerve), sometimes (46.15%) with hypoglossal component. Conclusions: These data suggest us to not consider myloglossus as a rare ana- tomical variant. -

The Mesozoic Era Alvarez, W.(1997)
Alles Introductory Biology: Illustrated Lecture Presentations Instructor David L. Alles Western Washington University ----------------------- Part Three: The Integration of Biological Knowledge Vertebrate Evolution in the Late Paleozoic and Mesozoic Eras ----------------------- Vertebrate Evolution in the Late Paleozoic and Mesozoic • Amphibians to Reptiles Internal Fertilization, the Amniotic Egg, and a Water-Tight Skin • The Adaptive Radiation of Reptiles from Scales to Hair and Feathers • Therapsids to Mammals • Dinosaurs to Birds Ectothermy to Endothermy The Evolution of Reptiles The Phanerozoic Eon 444 365 251 Paleozoic Era 542 m.y.a. 488 416 360 299 Camb. Ordov. Sil. Devo. Carbon. Perm. Cambrian Pikaia Fish Fish First First Explosion w/o jaws w/ jaws Amphibians Reptiles 210 65 Mesozoic Era 251 200 180 150 145 Triassic Jurassic Cretaceous First First First T. rex Dinosaurs Mammals Birds Cenozoic Era Last Ice Age 65 56 34 23 5 1.8 0.01 Paleo. Eocene Oligo. Miocene Plio. Ple. Present Early Primate First New First First Modern Cantius World Monkeys Apes Hominins Humans A modern Amphibian—the toad A modern day Reptile—a skink, note the finely outlined scales. A Comparison of Amphibian and Reptile Reproduction The oldest known reptile is Hylonomus lyelli dating to ~ 320 m.y.a.. The earliest or stem reptiles radiated into therapsids leading to mammals, and archosaurs leading to all the other reptile groups including the thecodontians, ancestors of the dinosaurs. Dimetrodon, a Mammal-like Reptile of the Early Permian Dicynodonts were a group of therapsids of the late Permian. Web Reference http://www.museums.org.za/sam/resource/palaeo/cluver/index.html Therapsids experienced an adaptive radiation during the Permian, but suffered heavy extinctions during the end Permian mass extinction. -

6. the Pharynx the Pharynx, Which Forms the Upper Part of the Digestive Tract, Consists of Three Parts: the Nasopharynx, the Oropharynx and the Laryngopharynx
6. The Pharynx The pharynx, which forms the upper part of the digestive tract, consists of three parts: the nasopharynx, the oropharynx and the laryngopharynx. The principle object of this dissection is to observe the pharyngeal constrictors that form the back wall of the vocal tract. Because the cadaver is lying face down, we will consider these muscles from the back. Figure 6.1 shows their location. stylopharyngeus suuperior phayngeal constrictor mandible medial hyoid bone phayngeal constrictor inferior phayngeal constrictor Figure 6.1. Posterior view of the muscles of the pharynx. Each of the three pharyngeal constrictors has a left and right part that interdigitate (join in fingerlike branches) in the midline, forming a raphe, or union. This raphe forms the back wall of the pharynx. The superior pharyngeal constrictor is largely in the nasopharynx. It has several origins (some texts regard it as more than one muscle) one of which is the medial pterygoid plate. It assists in the constriction of the nasopharynx, but has little role in speech production other than helping form a site against which the velum may be pulled when forming a velic closure. The medial pharyngeal constrictor, which originates on the greater horn of the hyoid bone, also has little function in speech. To some extent it can be considered as an elevator of the hyoid bone, but its most important role for speech is simply as the back wall of the vocal tract. The inferior pharyngeal constrictor also performs this function, but plays a more important role constricting the pharynx in the formation of pharyngeal consonants.