Zinc Nitrate Solution
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
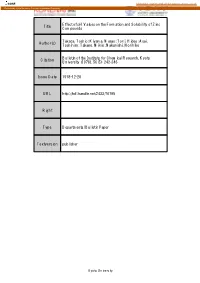
Title Effect of Ph Values on the Formation and Solubility Of
CORE Metadata, citation and similar papers at core.ac.uk Provided by Kyoto University Research Information Repository Effect of pH Values on the Formation and Solubility of Zinc Title Compounds Takada, Toshio; Kiyama, Masao; Torii, Hideo; Asai, Author(s) Toshihiro; Takano, Mikio; Nakanishi, Norihiko Bulletin of the Institute for Chemical Research, Kyoto Citation University (1978), 56(5): 242-246 Issue Date 1978-12-20 URL http://hdl.handle.net/2433/76795 Right Type Departmental Bulletin Paper Textversion publisher Kyoto University Bull.Inst. Chem.Res., Kyoto Univ., Vol. 56, No. 5, 1978 Effect of pH Values on the Formation and Solubility of Zinc Compounds Toshio TAKADA,Masao KIYAMA,Hideo TORII, Toshihiro AsAI* Mikio TAKANO**,and Norihiko NAKANISHI** ReceivedJuly 31, 1978 Aqueoussuspensions, prepared by mixingthe solution of NaOHand that ofzinc sulfate,chloride or nitrate,were subjected to agingat 25,50, and 70°C. Examinationof the productsby X-ray pow- der diffractionshowed that zinc oxide,basic zinc sulfate, chloride and nitrate are formeddepending mainlyon the pH. Their solubilitiesin the suspensionmedia with differentpH valueswere deter- mined at 25°C. INTRODUCTION In our laboratory, iron oxides and oxide hydroxides were prepared by wet methods such as the hydrolysis and slow oxidation of aqueous solutions of iron salts. The condi- tions for the formation of the oxides and oxide hydroxides') were reported together with their properties.2l The formation of a variety of products must be considered to be due to the difference in the nature -

Toxicological Profile for Zinc
TOXICOLOGICAL PROFILE FOR ZINC U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service Agency for Toxic Substances and Disease Registry August 2005 ZINC ii DISCLAIMER The use of company or product name(s) is for identification only and does not imply endorsement by the Agency for Toxic Substances and Disease Registry. ZINC iii UPDATE STATEMENT A Toxicological Profile for Zinc, Draft for Public Comment was released in September 2003. This edition supersedes any previously released draft or final profile. Toxicological profiles are revised and republished as necessary. For information regarding the update status of previously released profiles, contact ATSDR at: Agency for Toxic Substances and Disease Registry Division of Toxicology/Toxicology Information Branch 1600 Clifton Road NE Mailstop F-32 Atlanta, Georgia 30333 ZINC vi *Legislative Background The toxicological profiles are developed in response to the Superfund Amendments and Reauthorization Act (SARA) of 1986 (Public law 99-499) which amended the Comprehensive Environmental Response, Compensation, and Liability Act of 1980 (CERCLA or Superfund). This public law directed ATSDR to prepare toxicological profiles for hazardous substances most commonly found at facilities on the CERCLA National Priorities List and that pose the most significant potential threat to human health, as determined by ATSDR and the EPA. The availability of the revised priority list of 275 hazardous substances was announced in the Federal Register on November 17, 1997 (62 FR 61332). For prior versions of the list of substances, see Federal Register notices dated April 29, 1996 (61 FR 18744); April 17, 1987 (52 FR 12866); October 20, 1988 (53 FR 41280); October 26, 1989 (54 FR 43619); October 17, 1990 (55 FR 42067); October 17, 1991 (56 FR 52166); October 28, 1992 (57 FR 48801); and February 28, 1994 (59 FR 9486). -

UNIVERSITY of CALIFORNIA, IRVINE Synthesis of Gallium Zinc
UNIVERSITY OF CALIFORNIA, IRVINE Synthesis of Gallium Zinc Oxynitride Solid Solution by Flame Spray Pyrolysis THESIS submitted in partial satisfaction of the requirements for the degree of MASTER OF SCIENCE In Chemical and Biomolecular Engineering by Ewa Richard Chukwu Thesis Committee: Assistant Professor Erdem Sasmaz, Chair Assistant Professor Iryna Zenyuk Associate Professor Ali Mohraz 2020 © 2020 Ewa Richard Chukwu DEDICATION To The Almighty God who only him knows the end of a journey right from the beginning. Do not fear, for I am with you; Do not be dismayed for I am your God; I will strength you and help you; I will uphold you with my right hand of righteousness. Isaiah 49:10 ii TABLE OF CONTENTS LIST OF FIGURES……………………………………………………………. .iv LIST OF TABLES……….………………………………………………………vi ACKNOWLEDGEMENT………………………………….…………………...vii ABSTRACT……………………………..……………………………………....viii CHAPTER 1: Introduction………………………………………………..…........1 CHAPTER 2: Literature Review…………………………..………….........….......4 CHAPTER 3: Experimentation..............................................................................27 CHAPTER 4: Results and Discussions……………...…………………...............34 CHAPTER 5: Conclusion and Recommendation….….…..……………..............50 CHAPTER 6: Future Work …………………..………………….…...................53 REFERENCES………..…...……………………………………….…….............69 APPENDIX A.............………………………………………………..…..............83 APPENDIX B.................……………………………………………..…..............86 ii LIST OF FIGURES Fig.1: Flame synthesis versus wet chemistry……………………………………....5 -

“Inert” Ingredients Used in Organic Production
“Inert” Ingredients Used in Organic Production Terry Shistar, PhD A Beyond Pesticides Report he relatively few registered pesticides allowed in organic production are contained in product formulations with so-called “inert” ingredients that are not disclosed on the T product label. The “inerts” make up the powder, liquid, granule, or spreader/sticking agents in pesticide formulations. The “inerts” are typically included in products with natural or synthetic active pesticide ingredients recommended by the National Organic Standards Board (NOSB) and listed by the National Organic Program (NOP) on the National List of Allowed and Prohibited Substances. Any of the pesticides that meet the standards of public health and environmental protection and organic compatibility in the Organic Foods Production Act (OFPA) may contain “inert” ingredients. Because the standards of OFPA are different from those used by the U.S. Environmental Protection Agency (EPA) to regulate pesticides and given changes in how the agency categorizes inerts, the NOSB has adopted a series of recommendations since 2010 that established a substance review process as part of the sunset review. NOP has not followed through on the Board’s recommendations and, as a result, there are numerous materials in use that have not been subject to OFPA criteria. This report (i) traces the history of the legal requirements for review by the NOSB, (ii) identifies the universe of toxic and nontoxic materials that make of the category of “inerts” used in products permitted in organic production, and (iii) suggests a path forward to ensure NOSB compliance with OFPA and uphold the integrity of the USDA organic label. -

Cost Effective Zinc Nutrition Scott Johnson – UC Pomology Specialist Kearney Ag Center Hiawatha Nemaguard Peach Rootstocks and Zn Uptake December 2006 Shoot Zn
Cost Effective Zinc Nutrition Scott Johnson – UC Pomology Specialist Kearney Ag Center Hiawatha Nemaguard Peach Rootstocks and Zn Uptake December 2006 Shoot Zn 100 a Shoot 80 Zn (ppm) 60 40 b c c 20 0 Nemaguard Controller 5 Controller 9 Hiawatha Applying “Root Bags” with Sulfur, Urea and Zinc Sulfate to the Planting Hole in Spring 2006 25 a a b 20 b July Leaf Zn (ppm) 15 Site 1 10 5 0 2006 2007 Control 150 g Zn Sulfate Granular ZnO + Sulfur Material Added to Planting Hole of Peach (1.25 lbs/tree) 120 a 100 80 60 40 20 b July Leaf Zn (ppm) 0 Control Granular ZnO + S Treatment Granular ZnO + Sulfur Material Added to Planting Hole of Peach (1.25 lbs/tree) 2008 2009 120 a ) 100 ppm 80 Zn ( 60 a Leaf 40 y b Jul 20 b 0 Control Granular ZnO + S Treatment GranularGranular ZnOZnO ++ SulfurSulfur AddedAdded toto PlantingPlanting HoleHole ofof ZeeZee FireFire NectarineNectarine JanuaryJanuary 20092009 a 100 80 b 60 b 40 2009 Leaf Zn (ppm) y c Jul 20 0 1 1 0 /4# /2#1# Granular ZnO + S ZincZinc MaterialsMaterials AddedAdded toto PlantingPlanting HoleHole ofof FriarFriar // NemaguNemaguardard PlumsPlums FebruaryFebruary 20092009 a 120 100 b bc 80 60 cd cd 2009 Leaf Zn (ppm) y 40 d d Jul 20 d 0 1 1 1 1 1 UTC /4# /2#1# /4# /2#1# /8# Granular ZnO + S ZnO ZnSO4 ZincZinc MaterialsMaterials AddedAdded toto PlantingPlanting HoleHole ofof FriarFriar // NemaguNemaguardard PlumsPlums FebruaryFebruary 20092009 a 120 100 b bc 80 60 cd cd 2009 Leaf Zn (ppm) y 40 d d Jul 20 d 0 1 1 1 1 1 UTC /4# /2#1# /4# /2#1# /8# Granular ZnO + S ZnO ZnSO4 Nutrient Elements in -

Material Safety Data Sheet Zinc Nitrate Hexahydrate MSDS
He a lt h 2 1 Fire 1 2 0 Re a c t iv it y 0 Pe rs o n a l Pro t e c t io n E Material Safety Data Sheet Zinc nitrate hexahydrate MSDS Section 1: Chemical Product and Company Identification Product Name: Zinc nitrate hexahydrate Contact Information: Catalog Codes: SLZ1234 Sciencelab.com, Inc. 14025 Smith Rd. CAS#: 10196-18-6 Houston, Texas 77396 RTECS: ZH4775000 US Sales: 1-800-901-7247 International Sales: 1-281-441-4400 TSCA: TSCA 8(b) inventory: Zinc nitrate hexahydrate Order Online: ScienceLab.com CI#: Not available. CHEMTREC (24HR Emergency Telephone), call: Synonym: 1-800-424-9300 Chemical Formula: H12N2O12Zn International CHEMTREC, call: 1-703-527-3887 For non-emergency assistance, call: 1-281-441-4400 Section 2: Composition and Information on Ingredients Composition: Name CAS # % by Weight Zinc nitrate hexahydrate 10196-18-6 100 Toxicological Data on Ingredients: Zinc nitrate hexahydrate: ORAL (LD50): Acute: 1190 mg/kg [Rat]. 926 mg/kg [Mouse]. Section 3: Hazards Identification Potential Acute Health Effects: Hazardous in case of skin contact (irritant), of eye contact (irritant), of ingestion, of inhalation. Prolonged exposure may result in skin burns and ulcerations. Over-exposure by inhalation may cause respiratory irritation. Potential Chronic Health Effects: Hazardous in case of skin contact (irritant), of eye contact (irritant), of ingestion, of inhalation. CARCINOGENIC EFFECTS: Not available. MUTAGENIC EFFECTS: Not available. TERATOGENIC EFFECTS: Not available. DEVELOPMENTAL TOXICITY: Not available. The substance is toxic to mucous membranes. Repeated or prolonged exposure to the substance can produce target organs damage. Section 4: First Aid Measures Eye Contact: p. -

Life Cycle Assessment on Different Synthetic Routes of ZIF-8 Nanomaterials
energies Article Life Cycle Assessment on Different Synthetic Routes of ZIF-8 Nanomaterials Vasileios Ntouros 1,* , Ioannis Kousis 2,3, Dimitra Papadaki 1 , Anna Laura Pisello 2,3 and Margarita Niki Assimakopoulos 1 1 Group Building Environmental Research, Department of Physics, National and Kapodistrian University of Athens, University Campus, 157 84 Athens, Greece; [email protected] (D.P.); [email protected] (M.N.A.) 2 CIRIAF, Interuniversity Research Center, University of Perugia, Via G. Duranti 67, 06125 Perugia, Italy; [email protected] (I.K.); [email protected] (A.L.P.) 3 Department of Engineering, University of Perugia, Via G. Duranti 97, 06125 Perugia, Italy * Correspondence: [email protected] Abstract: In the last twenty years, research activity around the environmental applications of metal– organic frameworks has bloomed due to their CO2 capture ability, tunable properties, porosity, and well-defined crystalline structure. Thus, hundreds of MOFs have been developed. However, the impact of their production on the environment has not been investigated as thoroughly as their potential applications. In this work, the environmental performance of various synthetic routes of MOF nanoparticles, in particular ZIF-8, is assessed through a life cycle assessment. For this purpose, five representative synthesis routes were considered, and synthesis data were obtained based on available literature. The synthesis included different solvents (de-ionized water, methanol, dimethylformamide) as well as different synthetic steps (i.e., hours of drying, stirring, precursor). The Citation: Ntouros, V.; Kousis, I.; findings revealed that the main environmental weak points identified during production were: (a) the Papadaki, D.; Pisello, A.L.; use of dimethylformamide (DMF) and methanol (MeOH) as substances impacting environmental Assimakopoulos, M.N. -

New Insight Into the Mechanism of Cathodic Electrodeposition of Zinc Oxide Thin Films Onto Vitreous Carbon N
New insight into the mechanism of cathodic electrodeposition of zinc oxide thin films onto vitreous carbon N. Ait Ahmed, M. Eyraud, H. Hammache, F. Vacandio, S. Sam, N. Gabouze, P. Knauth, K. Pelzer, T. Djenizian To cite this version: N. Ait Ahmed, M. Eyraud, H. Hammache, F. Vacandio, S. Sam, et al.. New insight into the mechanism of cathodic electrodeposition of zinc oxide thin films onto vitreous carbon. Electrochimica Acta, Elsevier, 2014, 94, pp.238 - 244. 10.1016/j.electacta.2013.01.103. hal-01465928 HAL Id: hal-01465928 https://hal-amu.archives-ouvertes.fr/hal-01465928 Submitted on 21 Feb 2017 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. New insight into the mechanism of cathodic electrodeposition of zinc oxide thin films onto vitreous carbon. Ait Ahmed N.(1,3), Eyraud M.(2), Hammache H.(1), Vacandio F.(2), Sam S.(3), Gabouze N.(3), Knauth P. (2), Pelzer K.(2), Djenizian T.(4) (1) Université Abderrahmane Mira, Laboratoire de Technologie des matériaux et Génie des Procédés : Equipe d’électrochimie – Corrosion, Bejaia, 06000 Algérie. (2) Aix-Marseille Université - CNRS, UMR 7246 : Laboratoire Madirel, Equipe : Electrochimie des Matériaux- 13397 Marseille Cedex 20, France (3) Unité de Développement de la Technologie du Silicium (UDTS), B.P. -

Preparation and Thermal Chemical Properties of Compounds of Zinc Nitrate with Valine
Preparation and Thermal Chemical Properties of Compounds of Zinc Nitrate with Valine S. GAO*, S. CHEN, X. YANG, and Q. SHI Department of Chemistry, Shaanxi Key Laboratory of Physico-Inorganic Chemistry, Northwest University, Xi’an, 710069, P. R. China e-mail: [email protected] Received 12 August 2002 ◦ The solubility property of the Zn(NO3)2—Val—H2Osystemat25C in the whole composition range has been investigated by the semimicrophase equilibrium method. The corresponding phase diagrams and refractive index diagrams were constructed. From the phase equilibrium results, the in- congruently soluble compounds Zn(Val)(NO3)2 · 2H2O, Zn(Val)2(NO3)2 · H2O, and Zn(Val)3(NO3)2 · H2O were synthesized and characterized by IR, XRD, TG—DTG, as well as chemical and elemen- tal analyses. The constant-volume combustion energies of the compounds, ∆cE, determined by a precision rotating bomb calorimeter at 298.15 K, were (−10064.24 ± 5.41) J g−1,(−13523.98 ± 7.54) J g−1,and(−16116.13 ± 7.58) J g−1, respectively. The standard enthalpies of combustion for −1 these compounds, ∆cH,werecalculatedtobe(−3442.21 ± 2.25) kJ mol ,(−5971.2 ± 3.32) kJ mol−1,and(−9007.26 ± 4.24) kJ mol−1 while the standard enthalpies of formation were (−1017.34 ± 2.00) kJ mol−1,(−1742.93 ± 3.61) kJ mol−1,and(−2245.70 ± 4.73) kJ mol−1. The enthalpies of solution in condition of simulating human gastric juice (37 ◦C, pH = 1, the solution of hydrochloric acid), which were also measured by a microcalorimeter, were (22.17 ± 0.05) kJ mol−1, (14.89 ± 0.04) kJ mol−1,and(6.85± 0.07) kJ mol−1, respectively. -

Safety Data Sheet
SAFETY DATA SHEET 1. Identification Product identifier RNA 10% ZINC NITRATE SOLUTION Other means of identification None. Recommended use Ag Product - Plant Nutrition Recommended restrictions None known. Manufacturer/Importer/Supplier/Distributor information Manufacturer Company name RNA Address RNA 22312 Railroad Avenue San Joaquin, CA 93660 United States Telephone RNA (559) 693-4520 E-mail [email protected] Emergency phone number Chemtrec- Domestic (800) 424-9300 Chemtrec - International +1 703-741-5970 2. Hazard(s) identification Physical hazards Not classified. Health hazards Skin corrosion/irritation Category 1B Serious eye damage/eye irritation Category 1 Environmental hazards Not classified. OSHA defined hazards Not classified. Label elements Signal word Danger Hazard statement Causes severe skin burns and eye damage. Causes serious eye damage. Precautionary statement Prevention Do not breathe mist or vapor. Wash thoroughly after handling. Wear protective gloves/protective clothing/eye protection/face protection. Wear eye protection/face protection. Response If swallowed: Rinse mouth. Do NOT induce vomiting. If on skin (or hair): Take off immediately all contaminated clothing. Rinse skin with water/shower. If inhaled: Remove person to fresh air and keep comfortable for breathing. If in eyes: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. Immediately call a poison center/doctor. Wash contaminated clothing before reuse. Storage Store locked up. Disposal Dispose of -

Safety Data Sheet
SAFETY DATA SHEET Creation Date 16-Nov-2009 Revision Date 29-Jan-2021 Revision Number 2 SECTION 1: IDENTIFICATION OF THE SUBSTANCE/MIXTURE AND OF THE COMPANY/UNDERTAKING 1.1. Product identifier Product Description: Zinc nitrate hexahydrate Cat No. : A16282 Synonyms Nitric Acid, Zinc Salt, Hexahydrate CAS-No 10196-18-6 Molecular Formula N2 O6 Zn . 6 H2 O Reach Registration Number - 1.2. Relevant identified uses of the substance or mixture and uses advised against Recommended Use Laboratory chemicals. Uses advised against No Information available 1.3. Details of the supplier of the safety data sheet Company Thermo Fisher (Kandel) GmbH . Erlenbachweg 2 76870 Kandel Germany Tel: +49 (0) 721 84007 280 Fax: +49 (0) 721 84007 300 E-mail address [email protected] www.alfa.com Product safety Tel + +049 (0) 7275 988687-0 1.4. Emergency telephone number Carechem 24: +44 (0) 1235 239 670 (Multi-language emergency number) Poison Information Center Mainz www.giftinfo.uni-mainz.de Telephone: +49(0)6131/19240 Exclusively for customers in Austria: Poison Information Center (VIZ) Emergency call 0-24 clock: +43 1 406 43 43 Office hours: Monday to Friday, 8am to 4pm, tel: +43 1 406 68 98 SECTION 2: HAZARDS IDENTIFICATION 2.1. Classification of the substance or mixture CLP Classification - Regulation (EC) No 1272/2008 Physical hazards Oxidizing solids Category 2 (H272) ______________________________________________________________________________________________ ALFAAA16282 Page 1 / 11 SAFETY DATA SHEET Zinc nitrate hexahydrate Revision Date 29-Jan-2021 ______________________________________________________________________________________________ Health hazards Acute oral toxicity Category 4 (H302) Skin Corrosion/Irritation Category 2 (H315) Serious Eye Damage/Eye Irritation Category 2 (H319) Specific target organ toxicity - (single exposure) Category 3 (H335) Environmental hazards Chronic aquatic toxicity Category 2 (H411) Full text of Hazard Statements: see section 16 2.2. -

Dietary Zinc
NTP TECHNICAL REPORT ON THE TOXICOLOGY AND CARCINOGENESIS STUDY OF DIETARY ZINC (CAS NO. 5263-02-5) IN SPRAGUE DAWLEY RATS (Hsd:Sprague Dawley SD) (FEED STUDY) Scheduled Peer Review Date: July 13, 2017 NOTICE This DRAFT Technical Report is distributed solely for the purpose of predissemination peer review under the applicable information quality guidelines. It has not been formally disseminated by the NTP. It does not represent and should not be construed to represent NTP determination or policy. NTP TR 592 National Toxicology Program National Institutes of Health Public Health Service U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES FOREWORD The National Toxicology Program (NTP) is an interagency program within the Public Health Service (PHS) of the Department of Health and Human Services (HHS) and is headquartered at the National Institute of Environmental Health Sciences of the National Institutes of Health (NIEHS/NIH). Three agencies contribute resources to the program: NIEHS/NIH, the National Institute for Occupational Safety and Health of the Centers for Disease Control and Prevention (NIOSH/CDC), and the National Center for Toxicological Research of the Food and Drug Administration (NCTR/FDA). Established in 1978, the NTP is charged with coordinating toxicological testing activities, strengthening the science base in toxicology, developing and validating improved testing methods, and providing information about potentially toxic substances to health regulatory and research agencies, scientific and medical communities, and the public. The Technical Report series began in 1976 with carcinogenesis studies conducted by the National Cancer Institute. In 1981, this bioassay program was transferred to the NTP. The studies described in the Technical Report series are designed and conducted to characterize and evaluate the toxicologic potential, including carcinogenic activity, of selected substances in laboratory animals (usually two species, rats and mice).