Mmwr/Mmwr Continuingeducation.Html
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
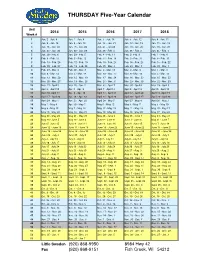
Thursday Calendar 20142018
THURSDAY Five-Year Calendar Unit 2014 20152016 2017 2018 Week # 1 Jan. 2 - Jan. 9 Jan. 1 - Jan. 8 Jan. 7 - Jan. 14 Jan. 5 - Jan. 12 Jan. 4 - Jan. 11 2 Jan. 9 - Jan. 16 Jan. 8 - Jan. 15 Jan. 14 - Jan. 21 Jan. 12 - Jan. 19 Jan. 11 - Jan. 18 3 Jan. 16 - Jan. 23 Jan. 15 - Jan. 22 Jan. 21 - Jan 28 Jan. 19 - Jan. 26 Jan. 18 - Jan. 25 4 Jan. 23 - Jan. 30 Jan. 22 - Jan. 29 Jan. 28 - Feb. 4 Jan. 26 - Feb. 2 Jan. 25 - Feb. 1 5 Jan. 30 - Feb. 6 Jan. 29 - Feb. 5 Feb. 4 - Feb. 11 Feb. 2- Feb. 9 Feb. 1 - Feb. 8 6 Feb. 6 - Feb. 13 Feb. 5 - Feb. 12 Feb. 11 - Feb. 18 Feb. 9 - Feb. 16 Feb. 8 - Feb. 15 7 Feb. 13 - Feb. 20 Feb. 12 - Feb. 19 Feb. 18 - Feb. 25 Feb. 16 - Feb. 23 Feb. 15 - Feb. 22 8 Feb. 20 - Feb. 27 Feb. 19 - Feb. 26 Feb. 25 - Mar. 3 Feb. 23 - Mar. 2 Feb. 22 - Mar. 1 9 Feb. 27 - Mar. 6 Feb. 26 - Mar. 5 Mar. 3 - Mar. 10 Mar. 2 - Mar. 9 Mar. 1 - Mar. 8 10 Mar. 6 - Mar. 13 Mar. 5 - Mar. 12 Mar. 10 - Mar. 17 Mar. 9 - Mar. 16 Mar. 5 - Mar. 15 11 Mar. 13 - Mar. 20 Mar. 12 - Mar. 19 Mar. 17 - Mar. 24 Mar. 16 - Mar. 23 Mar. 15 - Mar. 22 12 Mar. 20 - Mar. 27 Mar. 19 - Mar. 26 Mar. 24 - Mar. 31 Mar. 23 - Mar. 30 Mar. 22 - Mar. -
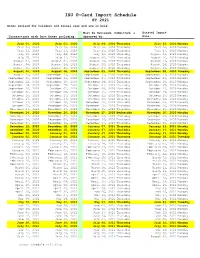
ISU P-Card Import Schedule FY 2021 Dates Revised for Holidays and Fiscal Year End Are in Bold
ISU P-Card Import Schedule FY 2021 Dates revised for holidays and fiscal year end are in bold. Must be Reviewed, Submitted, & Datatel Import Transactions with Post Dates including: Approved by: Date: July 01, 2020 July 03, 2020 July 09, 2020 Thursday July 13, 2020 Monday July 04, 2020 July 10, 2020 July 16, 2020 Thursday July 20, 2020 Monday July 11, 2020 July 17, 2020 July 23, 2020 Thursday July 27, 2020 Monday July 18, 2020 July 24, 2020 July 30, 2020 Thursday August 03, 2020 Monday July 25, 2020 July 31, 2020 August 06, 2020 Thursday August 10, 2020 Monday August 01, 2020 August 07, 2020 August 13, 2020 Thursday August 17, 2020 Monday August 08, 2020 August 14, 2020 August 20, 2020 Thursday August 24, 2020 Monday August 15, 2020 August 21, 2020 August 27, 2020 Thursday August 31, 2020 Monday August 22, 2020 August 28, 2020 September 03, 2020 Thursday September 08, 2020 Tuesday August 29, 2020 September 04, 2020 September 10, 2020 Thursday September 14, 2020 Monday September 05, 2020 September 11, 2020 September 17, 2020 Thursday September 21, 2020 Monday September 12, 2020 September 18, 2020 September 24, 2020 Thursday September 28, 2020 Monday September 19, 2020 September 25, 2020 October 01, 2020 Thursday October 05, 2020 Monday September 26, 2020 October 02, 2020 October 08, 2020 Thursday October 12, 2020 Monday October 03, 2020 October 09, 2020 October 15, 2020 Thursday October 19, 2020 Monday October 10, 2020 October 16, 2020 October 22, 2020 Thursday October 26, 2020 Monday October 17, 2020 October 23, 2020 October 29, 2020 Thursday -

2021 7 Day Working Days Calendar
2021 7 Day Working Days Calendar The Working Day Calendar is used to compute the estimated completion date of a contract. To use the calendar, find the start date of the contract, add the working days to the number of the calendar date (a number from 1 to 1000), and subtract 1, find that calculated number in the calendar and that will be the completion date of the contract Date Number of the Calendar Date Friday, January 1, 2021 133 Saturday, January 2, 2021 134 Sunday, January 3, 2021 135 Monday, January 4, 2021 136 Tuesday, January 5, 2021 137 Wednesday, January 6, 2021 138 Thursday, January 7, 2021 139 Friday, January 8, 2021 140 Saturday, January 9, 2021 141 Sunday, January 10, 2021 142 Monday, January 11, 2021 143 Tuesday, January 12, 2021 144 Wednesday, January 13, 2021 145 Thursday, January 14, 2021 146 Friday, January 15, 2021 147 Saturday, January 16, 2021 148 Sunday, January 17, 2021 149 Monday, January 18, 2021 150 Tuesday, January 19, 2021 151 Wednesday, January 20, 2021 152 Thursday, January 21, 2021 153 Friday, January 22, 2021 154 Saturday, January 23, 2021 155 Sunday, January 24, 2021 156 Monday, January 25, 2021 157 Tuesday, January 26, 2021 158 Wednesday, January 27, 2021 159 Thursday, January 28, 2021 160 Friday, January 29, 2021 161 Saturday, January 30, 2021 162 Sunday, January 31, 2021 163 Monday, February 1, 2021 164 Tuesday, February 2, 2021 165 Wednesday, February 3, 2021 166 Thursday, February 4, 2021 167 Date Number of the Calendar Date Friday, February 5, 2021 168 Saturday, February 6, 2021 169 Sunday, February -
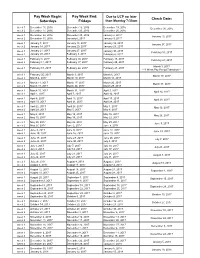
Pay Week Begin: Saturdays Pay Week End: Fridays Check Date
Pay Week Begin: Pay Week End: Due to UCP no later Check Date: Saturdays Fridays than Monday 7:30am week 1 December 10, 2016 December 16, 2016 December 19, 2016 December 30, 2016 week 2 December 17, 2016 December 23, 2016 December 26, 2016 week 1 December 24, 2016 December 30, 2016 January 2, 2017 January 13, 2017 week 2 December 31, 2016 January 6, 2017 January 9, 2017 week 1 January 7, 2017 January 13, 2017 January 16, 2017 January 27, 2017 week 2 January 14, 2017 January 20, 2017 January 23, 2017 January 21, 2017 January 27, 2017 week 1 January 30, 2017 February 10, 2017 week 2 January 28, 2017 February 3, 2017 February 6, 2017 week 1 February 4, 2017 February 10, 2017 February 13, 2017 February 24, 2017 week 2 February 11, 2017 February 17, 2017 February 20, 2017 March 3, 2017 week 1 February 18, 2017 February 24, 2017 February 27, 2017 ***1 Week Pay Period Transition*** week 1 February 25, 2017 March 3, 2017 March 6, 2017 March 17, 2017 week 2 March 4, 2017 March 10, 2017 March 13, 2017 week 1 March 11, 2017 March 17, 2017 March 20, 2017 March 31, 2017 week 2 March 18, 2017 March 24, 2017 March 27, 2017 week 1 March 25, 2017 March 31, 2017 April 3, 2017 April 14, 2017 week 2 April 1, 2017 April 7, 2017 April 10, 2017 week 1 April 8, 2017 April 14, 2017 April 17, 2017 April 28, 2017 week 2 April 15, 2017 April 21, 2017 April 24, 2017 week 1 April 22, 2017 April 28, 2017 May 1, 2017 May 12, 2017 week 2 April 29, 2017 May 5, 2017 May 8, 2017 week 1 May 6, 2017 May 12, 2017 May 15, 2017 May 26, 2017 week 2 May 13, 2017 May 19, 2017 May -

PRIVATE LAW 88-17-JULY 17, 1963 879 Private Law 88
77 STAT.] PRIVATE LAW 88-17-JULY 17, 1963 879 Private Law 88-14 AN ACT July 11, 1963 For the relief of Assnnta DiLella Codella. [H. R. 1736] Be it enacted hy the Senate and Houne of Representatives of the United States of America in Congress assemhled^ That, in the ad Assunta D. ministration of the Immigration and Nationality Act, Assunta DiLella Codella, Oodella may be classified as an eligible orphan within the meaning of section 101(b) (1) (F) of the Act, upon approval of a petition filed in 75 Stat, 6 50, her behalf by Mr. and Mrs. Joseph A. Codella, citizens of the United 8 use 1101. States, pursuant to section 205(b) of the Act, subject to all the condi 8 use 1155, tions in that section relating to eligible orphans. Approved July 11, 1963. Private Law 88-15 AN ACT July 11,1963 For the relief of Josephine Maria (Bonaccorso) Bowtell. [H, R, 3356] Be it enacted hy the Senate and House of Representatives of the United States of America, in Congress assembled^ That, in the admin Josephine M, istration of the Innnigration and Nationality Act, Josephine Maria Bowtell. (Bonaccoi-so) Bo\vtell shall be deemed to be within the purview of sect ion 354 (5) of that Act. 73 Stat, 274, Approved July 11, 1963. 8 use 1486, Private Law 88-16 AN ACT July 11, 1963 For the relief of Leroy Smallenherj^er, a referee in bankruptcy. [H, R, 4773] Be it enacted hy the Senate and House of Representatives of the United States of America in (Congress a^isemhled^ That, notwithstand Leroy ing any other provision of law, the Administrative Office of the Smallenberger. -

Julian Date Cheat Sheet for Regular Years
Date Code Cheat Sheet For Regular Years Day of Year Calendar Date 1 January 1 2 January 2 3 January 3 4 January 4 5 January 5 6 January 6 7 January 7 8 January 8 9 January 9 10 January 10 11 January 11 12 January 12 13 January 13 14 January 14 15 January 15 16 January 16 17 January 17 18 January 18 19 January 19 20 January 20 21 January 21 22 January 22 23 January 23 24 January 24 25 January 25 26 January 26 27 January 27 28 January 28 29 January 29 30 January 30 31 January 31 32 February 1 33 February 2 34 February 3 35 February 4 36 February 5 37 February 6 38 February 7 39 February 8 40 February 9 41 February 10 42 February 11 43 February 12 44 February 13 45 February 14 46 February 15 47 February 16 48 February 17 49 February 18 50 February 19 51 February 20 52 February 21 53 February 22 54 February 23 55 February 24 56 February 25 57 February 26 58 February 27 59 February 28 60 March 1 61 March 2 62 March 3 63 March 4 64 March 5 65 March 6 66 March 7 67 March 8 68 March 9 69 March 10 70 March 11 71 March 12 72 March 13 73 March 14 74 March 15 75 March 16 76 March 17 77 March 18 78 March 19 79 March 20 80 March 21 81 March 22 82 March 23 83 March 24 84 March 25 85 March 26 86 March 27 87 March 28 88 March 29 89 March 30 90 March 31 91 April 1 92 April 2 93 April 3 94 April 4 95 April 5 96 April 6 97 April 7 98 April 8 99 April 9 100 April 10 101 April 11 102 April 12 103 April 13 104 April 14 105 April 15 106 April 16 107 April 17 108 April 18 109 April 19 110 April 20 111 April 21 112 April 22 113 April 23 114 April 24 115 April -

Due Date Chart 201803281304173331.Xlsx
Special Event Permit Application Due Date Chart for Events from January 1, 2019 - June 30, 2020 If due date lands on a Saturday or Sunday, the due date is moved to the next business day Event Date 30 Calendar days 90 Calendar Days Tuesday, January 01, 2019 Sunday, December 02, 2018 Wednesday, October 03, 2018 Wednesday, January 02, 2019 Monday, December 03, 2018 Thursday, October 04, 2018 Thursday, January 03, 2019 Tuesday, December 04, 2018 Friday, October 05, 2018 Friday, January 04, 2019 Wednesday, December 05, 2018 Saturday, October 06, 2018 Saturday, January 05, 2019 Thursday, December 06, 2018 Sunday, October 07, 2018 Sunday, January 06, 2019 Friday, December 07, 2018 Monday, October 08, 2018 Monday, January 07, 2019 Saturday, December 08, 2018 Tuesday, October 09, 2018 Tuesday, January 08, 2019 Sunday, December 09, 2018 Wednesday, October 10, 2018 Wednesday, January 09, 2019 Monday, December 10, 2018 Thursday, October 11, 2018 Thursday, January 10, 2019 Tuesday, December 11, 2018 Friday, October 12, 2018 Friday, January 11, 2019 Wednesday, December 12, 2018 Saturday, October 13, 2018 Saturday, January 12, 2019 Thursday, December 13, 2018 Sunday, October 14, 2018 Sunday, January 13, 2019 Friday, December 14, 2018 Monday, October 15, 2018 Monday, January 14, 2019 Saturday, December 15, 2018 Tuesday, October 16, 2018 2019 Tuesday, January 15, 2019 Sunday, December 16, 2018 Wednesday, October 17, 2018 Wednesday, January 16, 2019 Monday, December 17, 2018 Thursday, October 18, 2018 Thursday, January 17, 2019 Tuesday, December 18, 2018 -

Resolution No.: 2021-______
RESOLUTION NO.: 2021-______ WHEREAS, the Mayor and Council of the City of Salem met from time to time in Executive Session for purposes authorized by the Illinois Open Meetings Act; and WHEREAS, as required by the Act, the City Clerk has kept written minutes of all such executive sessions; and WHEREAS, pursuant to the requirements of Public Act 85-1355, the Mayor and Council have met in closed session to review all closed session minutes; and WHEREAS, they have determined that a need for confidentiality still exists as to the executive session minutes from the meetings set forth on Schedule "A" attached hereto; and WHEREAS, they have further determined that the minutes of the meetings listed on Schedule "B" attached hereto no longer require confidential treatment and should be made available for public inspection; NOW, THEREFORE, BE IT RESOLVED BY THE MAYOR AND COUNCIL OF THE CITY OF SALEM, MARION COUNTY, ILLINOIS, as follows: SECTION 1: The Executive Session Minutes from those meetings set forth on Schedule "B" attached hereto are hereby released. SECTION 2: The City Clerk is hereby authorized and directed to make said minutes available for inspection and copying in accordance with the standing procedures of the Clerk's Office. 1 SECTION 3: This Resolution shall be in full force and effect from and after its passage. IN WITNESS WHEREOF, I have hereunto set my hand and caused the seal of the City of Salem, Illinois, to be affixed this 6th day of July, 2021. CITY OF SALEM, ILLINOIS MAYOR ATTEST: CITY CLERK 2 SCHEDULE A EXECUTIVE SESSION -

Federal Register/Vol. 85, No. 138/Friday, July 17, 2020/Rules
43436 Federal Register / Vol. 85, No. 138 / Friday, July 17, 2020 / Rules and Regulations Pursuant to the Regulatory Flexibility PART 300—USER FEES Electronic Availability Act (5 U.S.C. chapter 6), it is hereby Paragraph 1. The authority citation This document and additional certified that these final regulations will information concerning OFAC are not have a significant economic impact for part 300 continues to read as follows: available on OFAC’s website on a substantial number of small (www.treasury.gov/ofac). entities. The final regulations affect all Authority: 31 U.S.C. 9701. Background individuals who prepare or assist in Par. 2. Section 300.12 is amended by preparing all or substantially all of a tax revising paragraphs (b) and (d) to read On September 4, 2019, OFAC issued return or claim for refund for as follows: the Nicaragua Sanctions Regulations, 31 compensation. Only individuals, not CFR part 582 (84 FR 46440, September businesses, can have a PTIN. Thus, the § 300.13 Fee for obtaining a preparer tax 4, 2019) (the ‘‘Regulations’’) to economic impact of these regulations on identification number. implement Executive Order 13851 of any small entity generally will be a * * * * * November 27, 2018 (‘‘Blocking Property result of an individual tax return (b) Fee. The fee to apply for or renew of Certain Persons Contributing to the preparer who is required to have a PTIN a preparer tax identification number is Situation in Nicaragua’’) (E.O. 13851). owning a small business or a small $21 per year and is in addition to the The regulations were published in business otherwise employing an fee charged by the contractor. -

June 26, July 1*, July 10, July 17, July 24 Tri-County Championships
Family Last Name:________________________1 Hi Folks, As we anxiously await the start of the next swimming and diving seasons there are a few things I’d like to explain as you register. Please bring completed forms on 5/12. Page 3 must be filled out for each of your children who is participating. 1. Each family is responsible for working two meets and sending food for 2 pasta dinners . 2. For working a meet, just sign up for a date and we’ll put you where we need you. If you have a job you would like to work, please indicate it on the form. 3. We ask that you send enough food to serve 6-8. Ideally, you would sign up for one pasta and one salad or dessert. You may do both on the same night if you wish or send two pasta dishes or two salads, whichever you prefer. 4. The date(s) you choose for both working and cooking will be on both emailed to you and listed at the pool so you don’t forget. 5. Team swim suit (our suits are not available at a store) orders will take place on May 12 th as well as, orders for parent’s shirts. 6. If you are new to the team, Jenn Mason has volunteered to be the new and 8 and under team member liaison. If you have any questions please contact her at either [email protected] or 856-466-0028. 7. If your swimmer or diver has a sibling, who isn’t on the team, you may sign the sibling up to be part of our Wednesday parties by filling out the sibling form and paying the $10 fee. -

2021 Working Day Calendar-5 Day-Alternative Format
2021 Working Days Calendar – 5 day The Working Day Calendar is used to compute the estimated completion date of a contract. To use the calendar, find the start date of the contract, add the working days to the number of the calendar date (a number from 1 to 1000), and subtract 1, find that calculated number in the calendar and that will be the completion date of the contract Date Number of the Calendar Date Friday, January 1, 2021 Non-working Day Saturday, January 2, 2021 Non-working Day Sunday, January 3, 2021 Non-working Day Monday, January 4, 2021 938 Tuesday, January 5, 2021 939 Wednesday, January 6, 2021 940 Thursday, January 7, 2021 941 Friday, January 8, 2021 942 Saturday, January 9, 2021 Non-working Day Sunday, January 10, 2021 Non-working Day Monday, January 11, 2021 943 Tuesday, January 12, 2021 944 Wednesday, January 13, 2021 945 Thursday, January 14, 2021 946 Friday, January 15, 2021 947 Saturday, January 16, 2021 Non-working Day Sunday, January 17, 2021 Non-working Day Monday, January 18, 2021 Non-working Day Tuesday, January 19, 2021 948 Wednesday, January 20, 2021 949 Thursday, January 21, 2021 950 Friday, January 22, 2021 951 Saturday, January 23, 2021 Non-working Day Sunday, January 24, 2021 Non-working Day Monday, January 25, 2021 952 Tuesday, January 26, 2021 953 Wednesday, January 27, 2021 954 Thursday, January 28, 2021 955 Friday, January 29, 2021 956 Saturday, January 30, 2021 Non-working Day Sunday, January 31, 2021 Non-working Day Monday, February 1, 2021 957 Tuesday, February 2, 2021 958 Wednesday, February 3, -

Cal. C – Judge Boyd's Return Dates for Status Call @ 2:00
Cal. C – Judge Boyd’s Return Dates for Status Call @ 2:00 pm: Status set on June 15, 2020 are continued to August 11, 2020 Status set on June 16, 2020 are continued to August 12, 2020 Status set on June 17, 2020 are continued to August 13, 2020 Status set on June 18, 2020 are continued to August 14, 2020 Status set on June 19, 2020 are continued to August 17, 2020 Status set on June 22, 2020 are continued to August 18, 2020 Status set on June 23, 2020 are continued to August 19, 2020 Status set on June 24, 2020 are continued to August 20, 2020 Status set on June 25, 2020 are continued to August 21, 2020 Status set on June 26, 2020 are continued to August 24, 2020 Status set on June 29, 2020 are continued to August 25, 2020 Status set on June 30, 2020 are continued to August 26, 2020 Status set on July 1, 2020 are continued to August 27, 2020 Status set on July 2, 2020 are continued to August 28, 2020 Status set on July 6, 2020 are continued to August 31, 2020 Status set on July 7, 2020 are continued to September 1, 2020 Status set on July 8, 2020 are continued to September 2, 2020 Status set on July 9, 2020 are continued to September 3, 2020 Status set on July 10, 2020 are continued to September 4, 2020 Status set on July 13, 2020 are continued to September 8, 2020 Status set on July 14, 2020 are continued to September 9, 2020 Status set on July 15, 2020 are continued to September 10, 2020 Status set on July 16, 2020 are continued to September 11, 2020 Status set on July 17, 2020 are continued to September 14, 2020