Binary and Ternary Vanadium Oxides: General Overview, Physical Properties, and Photochemical Processes for Environmental Applications
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Vanadium Pentoxide and Other Inorganic Vanadium Compounds
This report contains the collective views of an international group of experts and does not necessarily represent the decisions or the stated policy of the United Nations Environment Programme, the International Labour Organization, or the World Health Organization. Concise International Chemical Assessment Document 29 VANADIUM PENTOXIDE AND OTHER INORGANIC VANADIUM COMPOUNDS Note that the layout and pagination of this pdf file are not identical to the printed CICAD First draft prepared by Dr M. Costigan and Mr R. Cary, Health and Safety Executive, Liverpool, United Kingdom, and Dr S. Dobson, Centre for Ecology and Hydrology, Huntingdon, United Kingdom Published under the joint sponsorship of the United Nations Environment Programme, the International Labour Organization, and the World Health Organization, and produced within the framework of the Inter-Organization Programme for the Sound Management of Chemicals. World Health Organization Geneva, 2001 The International Programme on Chemical Safety (IPCS), established in 1980, is a joint venture of the United Nations Environment Programme (UNEP), the International Labour Organization (ILO), and the World Health Organization (WHO). The overall objectives of the IPCS are to establish the scientific basis for assessment of the risk to human health and the environment from exposure to chemicals, through international peer review processes, as a prerequisite for the promotion of chemical safety, and to provide technical assistance in strengthening national capacities for the sound management -

Significance of Mineralogy in the Development of Flowsheets for Processing Uranium Ores
JfipwK LEACHING TIME REAGENTS TEMPERATURE FLOCCULANT CLARITY AREA COUNTER CURRENT DECANTATION It 21 21 J^^LJt TECHNICAL REPORTS SERIES No.19 6 Significance of Mineralogy in the Development of Flowsheets for Processing Uranium Ores \W# INTERNATIONAL ATOMIC ENERGY AGENCY, VIENNA, 1980 SIGNIFICANCE OF MINERALOGY IN THE DEVELOPMENT OF FLOWSHEETS FOR PROCESSING URANIUM ORES The following States are Members of the International Atomic Energy Agency: AFGHANISTAN HOLY SEE PHILIPPINES ALBANIA HUNGARY POLAND ALGERIA ICELAND PORTUGAL ARGENTINA INDIA QATAR AUSTRALIA INDONESIA ROMANIA AUSTRIA IRAN SAUDI ARABIA BANGLADESH IRAQ SENEGAL BELGIUM IRELAND SIERRA LEONE BOLIVIA ISRAEL SINGAPORE BRAZIL ITALY SOUTH AFRICA BULGARIA IVORY COAST SPAIN BURMA JAMAICA SRI LANKA BYELORUSSIAN SOVIET JAPAN SUDAN SOCIALIST REPUBLIC JORDAN SWEDEN CANADA KENYA SWITZERLAND CHILE KOREA, REPUBLIC OF SYRIAN ARAB REPUBLIC COLOMBIA KUWAIT THAILAND COSTA RICA LEBANON TUNISIA CUBA LIBERIA TURKEY CYPRUS LIBYAN ARAB JAMAHIRIYA UGANDA CZECHOSLOVAKIA LIECHTENSTEIN UKRAINIAN SOVIET SOCIALIST DEMOCRATIC KAMPUCHEA LUXEMBOURG REPUBLIC DEMOCRATIC PEOPLE'S MADAGASCAR UNION OF SOVIET SOCIALIST REPUBLIC OF KOREA MALAYSIA REPUBLICS DENMARK MALI UNITED ARAB EMIRATES DOMINICAN REPUBLIC MAURITIUS UNITED KINGDOM OF GREAT ECUADOR MEXICO BRITAIN AND NORTHERN EGYPT MONACO IRELAND EL SALVADOR MONGOLIA UNITED REPUBLIC OF ETHIOPIA MOROCCO CAMEROON FINLAND NETHERLANDS UNITED REPUBLIC OF FRANCE NEW ZEALAND TANZANIA GABON NICARAGUA UNITED STATES OF AMERICA GERMAN DEMOCRATIC REPUBLIC NIGER URUGUAY GERMANY, FEDERAL REPUBLIC OF NIGERIA VENEZUELA GHANA NORWAY VIET NAM GREECE PAKISTAN YUGOSLAVIA GUATEMALA PANAMA ZAIRE HAITI PARAGUAY ZAMBIA PERU The Agency's Statute was approved on 23 October 1956 by the Conference on the Statute of the IAEA held at United Nations Headquarters, New York; it entered into force on 29 July 1957. -

JAN Iia 20U T6T1/V
IN REPLY REFER TO: UNITED STATES DEPARTMENT OF THE INTERIOR GEOLOGICAL SURVEY WASHINGTON 25. D.C. November 19, 1956 AEC-193/7 Mr. Robert D. Nininger Assistant Director for Exploration Division of Bav Materials U* S. Atomic Energy Commission Washington 25, D. C, Dear Bobs Transmitted herewith are three copies of TEI-622, "The crystal chemistry and mineralogy of vanadium," by Ho-ward T. Evans, Jr. Me are asking Mr. Hosted to approve our plan to publish this report as a chapter of a Geological Survey professional paper on miner alogy and geochemistry of the ores of the Colorado Plateau. Aelrnovledg- ment of AEC sponsorship will be made in the introductory chapter* Sincerely yours, r ^ O U—— TV , Z^*i—w«__ ™~ W. H. Bradley Chief Geoldigist .JAN iia 20U T6T1/V Geology and 8$i:aeralQgy This document consists of k-2 pages* Series A» Howard T. Erans, Jr, Trace Elements Investigations Report 622 This preliminary report is distributed without editorial and technical review for conformity with official standards and nomenclature. It is not for public inspection or quotation* *This report concerns work done on behalf of the Division of Raw Materials of the U. S« Atomic Energy Commission* - TEI-622 AHD MIHERALQ6T Distribution (Series A) Ro. of copies Atomic Energy Commission, Washington .»**«»..*»..«*»..««..*... 2 Division of Rs¥ Materials, Albuquerque ,...****.*.«.»*.....*.. 1 Division of Raw Materials, Austin »«,..«.»...»*.»...*«..*...«» 1 Diylsion of Raw Materials, Butte *«*,.»».*..,*...».»......*.*. 1 Division of Raw Materials, Casper ............a............... 1 Division of Raw Ifeterials, Denver ,........»...».....«.,.*..... 1 Division of Raw Materials, Ishpeming .................a....... 1 Division of Raw Materials, Pnoenix ...a.....,....*........*... 1 Division of Eaw Materials, Rapid City ....................... -

Mineral Processing
Mineral Processing Foundations of theory and practice of minerallurgy 1st English edition JAN DRZYMALA, C. Eng., Ph.D., D.Sc. Member of the Polish Mineral Processing Society Wroclaw University of Technology 2007 Translation: J. Drzymala, A. Swatek Reviewer: A. Luszczkiewicz Published as supplied by the author ©Copyright by Jan Drzymala, Wroclaw 2007 Computer typesetting: Danuta Szyszka Cover design: Danuta Szyszka Cover photo: Sebastian Bożek Oficyna Wydawnicza Politechniki Wrocławskiej Wybrzeze Wyspianskiego 27 50-370 Wroclaw Any part of this publication can be used in any form by any means provided that the usage is acknowledged by the citation: Drzymala, J., Mineral Processing, Foundations of theory and practice of minerallurgy, Oficyna Wydawnicza PWr., 2007, www.ig.pwr.wroc.pl/minproc ISBN 978-83-7493-362-9 Contents Introduction ....................................................................................................................9 Part I Introduction to mineral processing .....................................................................13 1. From the Big Bang to mineral processing................................................................14 1.1. The formation of matter ...................................................................................14 1.2. Elementary particles.........................................................................................16 1.3. Molecules .........................................................................................................18 1.4. Solids................................................................................................................19 -
US Schedule for Internet V2
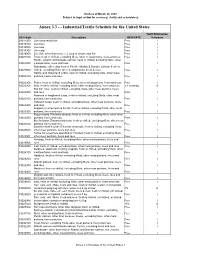
Draft as of March 23, 2007 Subject to legal review for accuracy, clarity and consistency. Annex 3.3 - - Industrial/Textile Schedule for the United States Tariff Elimination US 8 digit Description MFN RATE Schedule 03011000 Live ornamental fish Free I 03019100 Live trout Free I 03019200 Live eels Free I 03019300 Live carp Free I 03019900 Live fish, other than trout, eel, carp or ornamental fish Free I 03021100 Trout, fresh or chilled, excluding fillets, other meat portions, livers and roes Free I Pacific, Atlantic and Danube salmon, fresh or chilled, excluding fillets, other 03021200 meat portions, livers and roes Free I Salmonidae other than trout or Pacific, Atlantic & Danube salmon, fresh or 03021900 chilled, excluding fillets, other meat portions, livers & roes Free I Halibut and Greenland turbot, fresh or chilled, excluding fillets, other meat 03022100 portions, livers and roes Free I 03022200 Plaice, fresh or chilled, excluding fillets, other meat portions, livers and roes Free I 03022300 Sole, fresh or chilled, excluding fillets, other meat portions, livers and roes 1.1 cents/kg A Flat fish, nesi, fresh or chilled, excluding fillets, other meat portions, livers 03022900 and roes Free I Albacore or longfinned tunas, fresh or chilled, excluding fillets, other meat 03023100 portions, livers and roes Free I Yellowfin tunas, fresh or chilled, excluding fillets, other meat portions, livers 03023200 and roes Free I Skipjack or stripe-bellied bonito, fresh or chilled, excluding fillets, other meat 03023300 portions, livers and roes Free -

2012 Bmc Auction Specimens
A SAMPLER OF SELECTED 2017 BMC AUCTION SPECIMENS (2017 Auction Date is Saturday, 21 January) Volume 3 3+ Hematite [Fe 2O3] & Quartz [SiO2] Locality Cleator Moor Iron Mines Cleator Moor West Cumberland Iron Field Cumbria, England, UK Size 13.5 x 9.5 x 7.0 cm 1498 g Donated by Stonetrust Hematite Crystal System: Trigonal Photograph by Mike Haritos Physical Properties Transparency: Subtranslucent to opaque Mohs hardness: 6.5 Density: approx 5.3 gm/cm3 Streak: Red Luster: Metalic Vanadinite [Pb5(VO4)3Cl] var. Endlichite Locality Erupción Mine (Ahumada Mine) Los Lamentos Mountains (Sierra de Los Lamentos) Mun. de Ahumada Chihuahua, Mexico Size 12.0 x 9.5 x 7.0 cm 1134 g Donated by Stonetrust Crystal System: Hexagonal Physical Properties Transparency: Subtranslucent to opaque Mohs hardness: 3.5-4 Photograph by Mike Haritos Density: 6.8 to 7.1 gm/cm3 Streak: Brownish yellow Endlichite, Pb5([V, As]O4)3Cl, is the arsenic rich Luster: Adamantine variety of vanadinite with arsenic atoms (As) substituting for some of the vanadium (V) 2+ Dolomite [CaMg(CO3)2] & Chalcopyrite [CuFe S2] Locality Picher Field Tri-State District Ottawa Co. Oklahoma, USA Size 19.0 x 14.5 x 6.0 cm 1892 g Consigned with Reserve by Stonetrust Dolomite Crystal System: Trigonal Physical Properties Photograph by Mike Haritos Transparency: Transparent, Translucent, Opaque Mohs hardness: 3.5 to 4 Density: 2.8 to 2.9 gm/cm3 Streak: White Luster: Vitreous Calcite [CaCO3] Locality Mexico Size 15.5 x 12.8 x 6.2 cm 1074 g Donated by Stonetrust Calcite Crystal System: Trigonal Physical Properties Transparency: Transparent, Translucent Mohs hardness: 3 Density: 2.71 gm/cm3 Streak: White Luster: Vitreous, Sub-Vitreous, Photograph by Mike Haritos Resinous, Waxy, Pearly Quartz [SiO2], var. -

Adsorption of RNA on Mineral Surfaces and Mineral Precipitates
Adsorption of RNA on mineral surfaces and mineral precipitates Elisa Biondi1,2, Yoshihiro Furukawa3, Jun Kawai4 and Steven A. Benner*1,2,5 Full Research Paper Open Access Address: Beilstein J. Org. Chem. 2017, 13, 393–404. 1Foundation for Applied Molecular Evolution, 13709 Progress doi:10.3762/bjoc.13.42 Boulevard, Alachua, FL, 32615, USA, 2Firebird Biomolecular Sciences LLC, 13709 Progress Boulevard, Alachua, FL, 32615, USA, Received: 23 November 2016 3Department of Earth Science, Tohoku University, 2 Chome-1-1 Accepted: 15 February 2017 Katahira, Aoba Ward, Sendai, Miyagi Prefecture 980-8577, Japan, Published: 01 March 2017 4Department of Material Science and Engineering, Yokohama National University, 79-5 Tokiwadai, Hodogaya-ku, Yokohama This article is part of the Thematic Series "From prebiotic chemistry to 240-8501, Japan and 5The Westheimer Institute for Science and molecular evolution". Technology, 13709 Progress Boulevard, Alachua, FL, 32615, USA Guest Editor: L. Cronin Email: Steven A. Benner* - [email protected] © 2017 Biondi et al.; licensee Beilstein-Institut. License and terms: see end of document. * Corresponding author Keywords: carbonates; natural minerals; origins of life; RNA adsorption; synthetic minerals Abstract The prebiotic significance of laboratory experiments that study the interactions between oligomeric RNA and mineral species is difficult to know. Natural exemplars of specific minerals can differ widely depending on their provenance. While laboratory-gener- ated samples of synthetic minerals can have controlled compositions, they are often viewed as "unnatural". Here, we show how trends in the interaction of RNA with natural mineral specimens, synthetic mineral specimens, and co-precipitated pairs of synthe- tic minerals, can make a persuasive case that the observed interactions reflect the composition of the minerals themselves, rather than their being simply examples of large molecules associating nonspecifically with large surfaces. -

Aluminum-Vanadium System Donald Joseph Kenney Iowa State College
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1953 Aluminum-vanadium system Donald Joseph Kenney Iowa State College Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Physical Chemistry Commons Recommended Citation Kenney, Donald Joseph, "Aluminum-vanadium system " (1953). Retrospective Theses and Dissertations. 13302. https://lib.dr.iastate.edu/rtd/13302 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. NOTE TO USERS This reproduction is the best copy available. UMI mmMm-immim STSTEM BmaM S, Ktrmey A Mssertattioa aibaitted to the Sradttate Paeulty in fsKptial Fulfillffleat of the l®cpiir«a®ats tor the Degree of eocfoa or piiLosoFif Sabjecti aysiefil CheBdstry ^proved f Signature was redacted for privacy. In C2iarg®;!6f Ifejor Work Signature was redacted for privacy. Signature was redacted for privacy. Iowa State College 1953 UMI Number: DP12420 INFORMATION TO USERS The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleed-through, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. -

Cogjm.Search Apprais Radio Deposits Trace Elem Monthly Report Sept 1956.Pdf
RMO - 1081 UNITED STATES DEPARTMENT OF THE INTERIOR GEOLOGICAL SURVEY SEARCH FOR AND APPRAISAL OF RADIOACTIVE DEPOSITS TRACE ELEMENTS MONTHLY REPORT ( Prepared for U.S. Atomic Energy Commission ( Monthly Report September 1956 '\ -··..::7 REPRODUCED FROM BEST AVAILABLE CQ,JY Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed in this report, or represents that its use would not infringe privately owned rights. Reference therein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise, does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof. ~2- 3 CONTENTS Page Highlights of the Trace Elements Programeaeoooooeaeaooo••o••• 5 Geologic mappingoooooooooooooooooooooooo•••o••o•o••••••• 5 Colorado Plateau region8 by Ao Lo BrokaWooooooooooo 5 Central regionooooooooooooooooooooooooooo••o•••o••• 6 Maybell-Lay area9 Colorado, by M. J. Bergin •••• 6 Eastern regionoooooooooo•••o•o•oooo~ooooo•o•oo••••• 7 Hauch Chlmk 9 Pennsylvania, by tlarry Klemic •••• 7 Geologic topical StUdiSSooooooooooooooooOooooooooooooooo 8 Alaskaooooooooooooooooooooooooooooooooooooooooooooo -

Toxicological Profile for Vanadium
VANADIUM 107 4. CHEMICAL AND PHYSICAL INFORMATION 4.1 CHEMICAL IDENTITY Vanadium is a naturally occurring element that appears in group 5(B5) of the periodic table (Lide 2008). Vanadium is widely distributed in the earth’s crust at an average concentration of 100 ppm nd (approximately 100 mg/kg), similar to that of zinc and nickel (Byerrum 1991). Vanadium is the 22 most abundant element in the earth’s crust (Baroch 2006). Vanadium is found in about 65 different minerals; carnotite, roscoelite, vanadinite, and patronite are important sources of this metal along with bravoite and davidite (Baroch 2006, Lide 2008). It is also found in phosphate rock and certain ores and is present in some crude oils as organic complexes (Lide 2008). Table 4-1 lists common synonyms and other pertinent identification information for vanadium and representative vanadium compounds. 4.2 PHYSICAL AND CHEMICAL PROPERTIES Vanadium is a gray metal with a body-centered cubic crystal system. It is a member of the first transition series. Because of its high melting point, it is referred to as a refractory metal (Baroch 2006). When highly pure, it is a bright white metal that is soft and ductile. It has good structural strength and a low- fission neutron cross section. Vanadium has good corrosion resistance to alkalis, sulfuric and hydrochloric acid, and salt water; however, the metal oxidizes readily above 660 °C (Lide 2008). The chemistry of vanadium compounds is related to the oxidation state of the vanadium (Woolery 2005). Vanadium has oxidation states of +2, +3, +4, and +5. When heated in air at different temperatures, it oxidizes to a brownish black trioxide, a blue black tetraoxide, or a reddish orange pentoxide. -

Ferro Vanadium Production Business. Ferrovanadium Market Is Expected to Expand at a CAGR of 5.0% Between 2018 and 2028
www.entrepreneurindia.co Introduction Ferro Vanadium is an alloy which is formed by combining iron and vanadium with a vanadium content range of 35%-85%. Ferro Vanadium is a universal hardener, strengthener and anti-corrosive additive for steels like high-strength low-alloy (HSLA) steel, tool steels, as well as other ferrous-based products. Ferro vanadium belongs to the category of ferroalloy. Ferro vanadium is an alloy which is formed by combining iron and vanadium. Ferrovanadium contains 35% to 85% of vanadium depending on applications of the product in end-use industry. Ferro vanadium is an alloy material that is used in manufacturing of steel. It imparts desirable properties such as abrasion resistance, high temperature and hardenability. www.entrepreneurindia.co Ferro vanadium used for manufacturing of steel offers the end product with high stability against alkalis as well as acids such as sulphuric and hydrochloric acids. In addition, products containing ferro vanadium are at reduced risk to be susceptible to corrosion. Ferro vanadium also helps in reducing the overall weight of the material as well as increasing the overall tensile strength of the end product. In addition, it helps in promoting fine grain size and increasing hardenability through precipitation of nitrides and carbides. Ferro vanadium is manufactured using an electric arc furnace in which scrap iron is melted initially and then it is combined with the mixture of aluminum as well as flux such as calcium fluoride and calcium oxide. It is usually supplied in pallet boxes or in shrink wrapped in super bags. www.entrepreneurindia.co Ferro Vanadium is produced from Vanadium Sludge & usually available in the range with V: 50-85%. -

Polyanionic Cathode Materials for Sodium-Ion Batteries
Polyanionic cathode materials for sodium-ion batteries Zur Erlangung des akademischen Grades eines DOKTORS DER NATURWISSENSCHAFTEN (Dr. rer. nat.) der KIT-Fakultät für Chemie und Biowissenschaften des Karlsruher Instituts für Technologie (KIT) genehmigte DISSERTATION von Herr Huang Zhang KIT-Dekan: Prof. Dr. Reinhard Fischer Referent: Prof. Dr. Stefano Passerini Korreferent: Prof. Dr. Helmut Ehrenberg Tag der mündlichen Prüfung: 13 Dezember 2018 To my family “A journey of a thousand miles begins with a single step.” (Lao-Tzu) Acknowledgments First of all, I would like to thank my advisor, Prof. Stefano Passerini, for giving me the chance to explore in sodium-ion batteries. I am grateful that he challenged me to meet his high scientific and communication standards, for his flexibility in allowing me to pursue what I found interesting, and for trusting me to solve problems with an independent approach. I really appreciate the enjoyable environment to work in Electrochemistry I group. I am also very grateful to Prof. Helmut Ehrenberg for being the second examiner of my thesis, especially for his fruitful discussion. I would like to thank Dr. Daniel Buchholz and Dr. Ivana Hasa with whom I was working in the sodium-ion battery research field. I am really grateful to their help on my work. Dr. Alberto Varzi, Dr. Sangsik Jeong, Dr. Diogo Vieira Carvalho, Lucas Lodovico, and Jakob Asenbauer are also acknowledged. Without their support this thesis would not be possible. I would like to express my gratitude to Dr. Dominic Bresser for his critical review and correction on the thesis. Francesco Liberale from Polytechnic University of Milan is also acknowledged for his critical discussion on the thesis.