Uptake of Fe-Fraxetin Complexes, an IRT1 Independent Strategy for Iron Acquisition In
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Suspect and Target Screening of Natural Toxins in the Ter River Catchment Area in NE Spain and Prioritisation by Their Toxicity
toxins Article Suspect and Target Screening of Natural Toxins in the Ter River Catchment Area in NE Spain and Prioritisation by Their Toxicity Massimo Picardo 1 , Oscar Núñez 2,3 and Marinella Farré 1,* 1 Department of Environmental Chemistry, IDAEA-CSIC, 08034 Barcelona, Spain; [email protected] 2 Department of Chemical Engineering and Analytical Chemistry, University of Barcelona, 08034 Barcelona, Spain; [email protected] 3 Serra Húnter Professor, Generalitat de Catalunya, 08034 Barcelona, Spain * Correspondence: [email protected] Received: 5 October 2020; Accepted: 26 November 2020; Published: 28 November 2020 Abstract: This study presents the application of a suspect screening approach to screen a wide range of natural toxins, including mycotoxins, bacterial toxins, and plant toxins, in surface waters. The method is based on a generic solid-phase extraction procedure, using three sorbent phases in two cartridges that are connected in series, hence covering a wide range of polarities, followed by liquid chromatography coupled to high-resolution mass spectrometry. The acquisition was performed in the full-scan and data-dependent modes while working under positive and negative ionisation conditions. This method was applied in order to assess the natural toxins in the Ter River water reservoirs, which are used to produce drinking water for Barcelona city (Spain). The study was carried out during a period of seven months, covering the expected prior, during, and post-peak blooming periods of the natural toxins. Fifty-three (53) compounds were tentatively identified, and nine of these were confirmed and quantified. Phytotoxins were identified as the most frequent group of natural toxins in the water, particularly the alkaloids group. -

Scopoletin 8-Hydroxylase: a Novel Enzyme Involved in Coumarin
bioRxiv preprint doi: https://doi.org/10.1101/197806; this version posted October 4, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Scopoletin 8-hydroxylase: a novel enzyme involved in coumarin biosynthesis and iron- 2 deficiency responses in Arabidopsis 3 4 Running title: At3g12900 encodes a scopoletin 8-hydroxylase 5 6 Joanna Siwinska1, Kinga Wcisla1, Alexandre Olry2,3, Jeremy Grosjean2,3, Alain Hehn2,3, 7 Frederic Bourgaud2,3, Andrew A. Meharg4, Manus Carey4, Ewa Lojkowska1, Anna 8 Ihnatowicz1,* 9 10 1Intercollegiate Faculty of Biotechnology of University of Gdansk and Medical University of 11 Gdansk, Abrahama 58, 80-307 Gdansk, Poland 12 2Université de Lorraine, UMR 1121 Laboratoire Agronomie et Environnement Nancy- 13 Colmar, 2 avenue de la forêt de Haye 54518 Vandœuvre-lès-Nancy, France; 3INRA, UMR 14 1121 Laboratoire Agronomie et Environnement Nancy-Colmar, 2 avenue de la forêt de Haye 15 54518 Vandœuvre-lès-Nancy, France; 16 4Institute for Global Food Security, Queen’s University Belfast, David Keir Building, Malone 17 Road, Belfast, UK; 18 19 [email protected] 20 [email protected] 21 [email protected] 22 [email protected] 23 [email protected] 24 [email protected] 25 [email protected] 26 [email protected] 27 [email protected] 28 *Correspondence: [email protected], +48 58 523 63 30 29 30 The date of submission: 02.10.2017 31 The number of figures: 9 (Fig. -

Biosynthesis of Daphnetin in Daphne Mezereum L.*
Biosynthesis of Daphnetin in Daphne mezereum L.* Stewart A. Brown Department of Chemistry, Trent University, Peterborough, Ont., Canada K9J 7B8 Z. Naturforsch. 41c, 247—252 (1986); received November 4, 1985 Biosynthesis, Coumarins, Daphne mezereum, Daphnetin Shoots of Daphne mezereum synthesized daphnetin (7,8-dihydroxycoumarin) more efficiently from [2-l4C]umbelliferone (7-hydroxycoumarin) than from [2-l4C]p-coumaric acid, and [2-14C]caf- feic acid was more poorly utilized still. These findings do not support the idea of derivation of daphnetin via hydroxylation of the caffeic acid ring at the 2 position, followed by lactone ring formation; instead they are consistent with the concept of daphnetin formation by an additional hydroxylation of umbelliferone at C-8. Umbelliferone was recovered with little l4C dilution from emulsin-hydrolysed extracts of shoots fed labelled umbelliferone, and TLC of extracts from un treated shoots revealed two substances yielding umbelliferone on hydrolysis. Evidence is pre sented from TLC and HPLC analysis that one of these is skimmin (7-O-ß-D-glucosylumbel- liferone), not previously reported from Daphne. The tracer experiments further support the theory that umbelliferone is the general precursor of coumarins bearing two or more hydroxyl functions on the aromatic ring. Introduction (Thymelaeaceae), a hardy perennial shrub, where it Approximately 70 coumarins with the 7,8 oxygen occurs together with its 7-ß-D-glucoside, daphnin ation pattern' have been reported to be naturally (6b) and daphnetin glucoside (6c), the 8-O-ß-D- occurring [1], over half being furanocoumarins de g lu c o s y la te d is o m e r [1]. -

A Detailed Review on Morphotaxonomy And
Acta Scientific Microbiology (ISSN: 2581-3226) Review Article Volume 1 Issue 6 June 2018 Skimmia anquetilia N.P. Taylor and Airy Shaw A Detailed Review on Morphotaxonomy and Chemoprofiling of Saduf Nissar1, Neelofar Majid1, Aabid M Rather1*, Irshad A Nawchoo1 and GG Mohi-Ud-Din2 1Plant Reproductive Biology, Genetic Diversity and Phytochemistry Research Laboratory, Department of Botany, University of Kashmir, Srinagar, India 2Department of Botany, Government Degree College for Women, Sopore, Baramullah, India *Corresponding Author: Aabid M Rather, Plant Reproductive Biology, Genetic Diversity and Phytochemistry Research Laboratory, DepartmentReceived: April of Botany, 20, 2018; University Published: of Kashmir, May 28, Srinagar, 2018 India. Abstract - In recent times, medicinal plants have attracted huge attention due to their diverse range of biological and therapeutic proper Skimmia ties. Evidences have been accumulated since ages to demonstrate promising potential of medicinal plants used in various traditional, anquetilia complementary, and alternative systems with the ever-increasing interest of today’s population towards natural products, Rutaceae N.P. Taylor and Airy Shaw emerged out to be one of the most eye-catching plant bearing multiple medicinal properties. It is a perennial aromatic evergreen shrub belonging to family . Pharmacological studies have demonstrated significant action - of different extracts as antimicrobial, anti-inflammatory agents, among others, supporting some of its popular uses. An attempt has S. anquetilia been made in this review article to provide an up-to-date overview of the morphological parameters, taxonomic features, distribu tion pattern, traditional uses, as well as the phytochemistry and biological activities of . The present review provides insights for future research aiming for both ethnopharmacological validation of its popular use and its exploration as a new source ofKeywords herbal drugs: Skimmia and/or anquetilia; bioactive Rutaceaenatural products. -

Morning Glory Systemically Accumulates Scopoletin And
Morning Glory Systemically Accumulates Scopoletin and Scopolin after Interaction with Fusarium oxysporum Bun-ichi Shimizua,b,*, Hisashi Miyagawaa, Tamio Uenoa, Kanzo Sakatab, Ken Watanabec, and Kei Ogawad a Graduate School of Agriculture, Kyoto University, Kyoto 606Ð8502, Japan b Institute for Chemical Research, Kyoto University, Uji 611-0011, Japan. Fax: +81-774-38-3229. E-mail: [email protected] c Ibaraki Agricultural Center, Ibaraki 311Ð4203, Japan d Kyushu National Agricultural Experiment Station, Kumamoto 861Ð1192, Japan * Author for correspondence and reprint requests Z. Naturforsch. 60c,83Ð90 (2005); received September 14/October 29, 2004 An isolate of non-pathogenic Fusarium, Fusarium oxysporum 101-2 (NPF), induces resis- tance in the cuttings of morning glory against Fusarium wilt caused by F. oxysporum f. sp. batatas O-17 (PF). The effect of NPF on phenylpropanoid metabolism in morning glory cuttings was studied. It was found that morning glory tissues responded to treatment with NPF bud-cell suspension (108 bud-cells/ml) with the activation of phenylalanine ammonia- lyase (PAL). PAL activity was induced faster and greater in the NPF-treated cuttings com- pared to cuttings of a distilled water control. High performance liquid chromatography analy- sis of the extract from tissues of morning glory cuttings after NPF treatment showed a quicker induction of scopoletin and scopolin synthesis than that seen in the control cuttings. PF also the induced synthesis of these compounds at 105 bud-cells/ml, but inhibited it at 108 bud- cells/ml. Possibly PF produced constituent(s) that elicited the inhibitory effect on induction of the resistance reaction. -

Accumulation and Secretion of Coumarinolignans and Other Coumarins in Arabidopsis Thaliana Roots in Response to Iron Deficiency
Accumulation and Secretion of Coumarinolignans and other Coumarins in Arabidopsis thaliana Roots in Response to Iron Deficiency at High pH Patricia Siso-Terraza, Adrian Luis-Villarroya, Pierre Fourcroy, Jean-Francois Briat, Anunciacion Abadia, Frederic Gaymard, Javier Abadia, Ana Alvarez-Fernandez To cite this version: Patricia Siso-Terraza, Adrian Luis-Villarroya, Pierre Fourcroy, Jean-Francois Briat, Anunciacion Aba- dia, et al.. Accumulation and Secretion of Coumarinolignans and other Coumarins in Arabidopsis thaliana Roots in Response to Iron Deficiency at High pH. Frontiers in Plant Science, Frontiers, 2016, 7, pp.1711. 10.3389/fpls.2016.01711. hal-01417731 HAL Id: hal-01417731 https://hal.archives-ouvertes.fr/hal-01417731 Submitted on 15 Dec 2016 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. fpls-07-01711 November 21, 2016 Time: 15:23 # 1 ORIGINAL RESEARCH published: 23 November 2016 doi: 10.3389/fpls.2016.01711 Accumulation and Secretion of Coumarinolignans and other Coumarins in Arabidopsis thaliana Roots in Response to Iron Deficiency at -
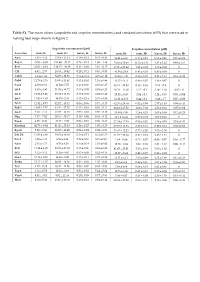
(Scopoletin and Scopolin Concentrations) and Standard Deviations (±SD) That Were Used in Making Heat Maps Shown in Figure 2
Table S1. The mean values (scopoletin and scopolin concentrations) and standard deviations (±SD) that were used in making heat maps shown in Figure 2. Scopoletin concentration [µM] Scopolin concentration [µM] Accession roots, H- roots, H+ leaves, H- leaves, H+ roots, H- roots, H+ leaves, H- leaves, H+ An-1 4.91 ± 4.12 15.19 ± 11.34 0.10 ± 0.12 0.45 ± 0.24 14.81 ± 4.05 0.13 ± 0.12 0.68 ± 0.41 0.05 ± 0.08 Bay-0 41.93 ± 6.58 151.90 ± 30.12 0.29 ± 0.18 1.99 ± 1.46 38.95 ± 10.69 07.15 ± 6.36 3.81 ± 1.22 0.88 ± 1.53 Br-0 35.01 ± 9.48 139.27 ± 49.38 0.31 ± 0.09 2.61 ± 0.27 67.26 ± 23.82 1.22 ± 0.08 1.65 ± 0.08 0 C24 4.92 ± 2.67 16.63 ± 10.42 0.20 ± 0.11 0.83 ± 0.55 41.36 ± 24.8 0.43 ± 0.19 0.85 ± 0.59 0 Can-0 3.14 ± 1.89 64.03 ± 84.91 0.14 ± 0.14 0.37 ± 0.12 34.63 ± 4.85 0.56 ± 0.05 2.23 ± 1.24 0.19 ± 0.33 Col-0 11.79 ± 3.16 29.60 ± 11.41 0.31 ± 0.41 1.16 ± 0.99 11.47 ± 3.41 0.46 ± 0.07 1.48 ± 0.67 0 Cvi-1 4.58 ± 0.62 11.54 ± 7.57 0.19 ± 0.10 0.61 ± 0.27 35.41 ± 32.83 0.35 ± 0.16 2.15 ± 0.8 0 Eil-0 8.03 ± 4.90 21.55 ± 14.72 0.15 ± 0.03 0.53 ± 0.23 28.24 ± 11.10 1.12 ± 0.2 2.19 ± 1.16 0.02 ± 0 Eri-0 12.86 ± 3.96 23.84 ± 13.33 0.13 ± 0.09 0.68 ± 0.48 20.55 ± 8.45 2.04 ± 0.3 2.24 ± 0.93 0.03 ± 0.04 Est-1 14.85 ± 1.63 36.79 ± 2.44 0.42 ± 0.19 2.01 ± 0.86 32.22 ±12.66 0.44 ± 0.2 6.48 ± 3.7 0.07 ± 0.09 Fei-0 12.82 ± 8.73 52.02 ± 15.32 0.09 ± 0.06 0.73 ± 0.37 62.36 ± 28.64 0.52 ± 0.08 2.77 ± 1.53 0.08 ± 0.13 Fuk-1 13.06 ± 7.87 44.36 ± 47.37 0.13 ± 0.01 0.62 ± 0.11 29.23 ± 27.52 8.09 ± 7.02 1.58 ± 0.62 0.05 ± 0.09 Ga-0 3.40 ± 1.11 19.67 ± 12.65 0.03 -

Isolated and Identified As Scopoletin (6-Methoxy-7-Hydroxycourmarin; 6-Methoxy- 7-Hydroxy-1,2-Benzopyrone)
36 BOTANY: SKOOG AND MONTALDI PRoc. N. A. S. 22 Lake G. R., P. McCutchun, R. Van Meter, and J. C. Neel, Anal. Chem., 23, 1634 (1951). 23 Mackinney, G., J. Biol. Chem., 140, 315 (1941). 24 Hartree, B. E. F., in Modern Methods of Plant Analysis, ed. K. Paech, and M. V. Tracey (Berlin: Springer-Verlag, 1955), Vol. IV, p. 241. 25 Pharmacopeia of the United States, Fifteenth Revision (American Pharmaceutical Association, 1955), p. 186. 26 Ford, J. E., Brit. J. Nutrition, 7, 299 (1953). 27 Saltzman, B. E., Anal. Chem., 27, 284 (1955). " Galston, A. W., and S. M. Siegel, Science, 120, 1070 (1954). 29 Thimann, K. V., Amer. J. Bot., 43, 241 (1956). 30 Porter, J. W., Proc. Nutrition Soc. (Gr. Brit.), 12, 106 (1953). A UXIN-KINETIN INTERACTION REGULATING THE SCOPOLETIN AND SCOPOLIN LEVELS IN TOBACCO TISSUE CULTURES BY FOLKE SKOOG AND EDGARDO MONTALDI* DEPARTMENT OF BOTANY, UNIVERSITY OF WISCONSIN Communicated November 17, 1960 Tobacco tissue cultured in vitro has been observed to release a strongly ultra- violet fluorescent material into the agar medium. The intensity of the fluores- cence varies with the composition of the medium and the age and other conditions of the cultures. It is especially striking in the presence of high concentrations of 3- indoleacetic acid (IAA). A major fraction of the fluorescent material has been isolated and identified as scopoletin (6-methoxy-7-hydroxycourmarin; 6-methoxy- 7-hydroxy-1,2-benzopyrone). Influences of auxins and kinetin on its formation, its liberation from a glycosidic bound form, and its release into the medium will be reported. -

Phenylpropanoids
Phenylpropanoids The phenylpropanoids are a diverse family of organic compounds that are synthesized by plants from the amino acids phenylalanine and tyrosine. Their name is derived from the six-carbon, aromatic phenyl group and the three-carbon propene tail of cinnamic acid, which is synthesized from phenylalanine in the first step of phenylpropanoid biosynthesis. Phenylpropanoids are found throughout the plant kingdom, where they serve as essential components of a number of structural polymers, provide protection from ultraviolet light, defend against herbivores and pathogens, and mediate plant-pollinator interactions as floral pigments and scent compounds. Concentrations of phenylpropanoids within plants are also altered by changes in resource availability. www.MedChemExpress.com 1 Phenylpropanoids Inhibitors & Modulators (+)-Columbianetin (+)-Columbianetin acetate ((S)-Columbianetin) Cat. No.: HY-N0363 ((S)-Columbianetin acetate) Cat. No.: HY-N0363A (+)-Columbianetin is an isomer of Columbianetin. (S)-Columbianetin acetate is an isomer of Columbianetin is a phytoalexin associated with Columbianetin. Columbianetin is a phytoalexin celery (Apium graveolens) resistance to associated with celery (Apium graveolens) pathogens during storage. Columbianetin exhibits resistance to pathogens during storage. excellent anti-fungal and anti-inflammatory Columbianetin exhibits excellent anti-fungal and activity. anti-inflammatory activity. Purity: >98% Purity: >98% Clinical Data: No Development Reported Clinical Data: No Development Reported Size: 5 mg, 10 mg, 20 mg Size: 5 mg, 10 mg, 20 mg (+)-Guaiacin (+)-Peusedanol Cat. No.: HY-N2247A Cat. No.: HY-N6063 (+)-Guaiacin is a compound extracted of the bark (+)-Peusedanol is a coumarin isolated from of Machilus wangchiana Chun. (Lauraceae). Peucedanumjaponicum. (+)-Guaiacin shows potent in vitro activities against the release of β-glucuronidase in rat polymorphonuclear leukocytes (PMNs) induced by platelet-activating factor (PAF) . -

Identification and Functional Characterization of the First Two
Identification and functional characterization of the first two aromatic prenyltransferases implicated in the biosynthesis of furanocoumarins and prenylated coumarins in two plant families: Rutaceae and Apiaceae Fazeelat Karamat To cite this version: Fazeelat Karamat. Identification and functional characterization of the first two aromatic prenyl- transferases implicated in the biosynthesis of furanocoumarins and prenylated coumarins in two plant families: Rutaceae and Apiaceae. Agronomy. Université de Lorraine, 2013. English. NNT : 2013LORR0029. tel-01749560 HAL Id: tel-01749560 https://hal.univ-lorraine.fr/tel-01749560 Submitted on 29 Mar 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. AVERTISSEMENT Ce document est le fruit d'un long travail approuvé par le jury de soutenance et mis à disposition de l'ensemble de la communauté universitaire élargie. Il est soumis à la propriété intellectuelle de l'auteur. Ceci implique une obligation de citation et de référencement lors de l’utilisation de ce document. D'autre part, toute contrefaçon, plagiat, -

Natural Compounds, Fraxin and Chemicals Structurally Related to Fraxin Protect Cells from Oxidative Stress
EXPERIMENTAL and MOLECULAR MEDICINE, Vol. 37, No. 5, 436-446, October 2005 Natural compounds, fraxin and chemicals structurally related to fraxin protect cells from oxidative stress Wan Kyunn Whang1, Hyung Soon Park2, genes expressed differentially by fraxin and to InHye Ham1, Mihyun Oh1, compare antioxidative effect of fraxin with its struc- Hong Namkoong3, Hyun Kee Kim3, turally related chemicals. Of the coumarins, protective 2 4 effects of fraxin against cytotoxicity induced by H2O2 Dong Whi Hwang , Soo Young Hur , were examined in human umbilical vein endothelial 4 5 Tae Eung Kim , Yong Gyu Park , cells (HUVECs). Fraxin showed free radical scavenging Jae-Ryong Kim6 and Jin Woo Kim3,4,7 effect at high concentration (0.5 mM) and cell protective effect against H2O2-mediated oxidative stress. Fraxin 1 College of Pharmacy, Chung-Ang University recovered viability of HUVECs damaged by H2O2- 221, Heukseok-dong, Dongjak-gu treatment and reduced the lipid peroxidation and the Seoul 156-861, Korea internal reactive oxygen species level elevated by H2O2 2KeyGene Life Science Institute treatment. Differential display reverse transcrip- KeyGene Science, Corp. tion-PCR revealed that fraxin upregulated antiapo- Ansan, Gyeonggido 425-791, Korea ptotic genes (clusterin and apoptosis inhibitor 5) and 3Molecular Genetic Laboratory tumor suppressor gene (ST13). Based on structural Research Institute of Medical Science similarity comparing with fraxin, seven chemicals, 4Department of Obstetrics and Gynecology fraxidin methyl ether (29.4% enhancement of viability), 5Department of Biostatistics prenyletin (26.4%), methoxsalen (20.8 %), diffratic acid College of Medicine (19.9%), rutoside (19.1%), xanthyletin (18.4%), and The Catholic University of Korea kuhlmannin (18.2%), enhanced more potent cell via- Seoul 137-040, Korea bility in the order in comparison with fraxin, which 6Department of Biochemistry and Molecular Biology showed only 9.3% enhancement of cell viability. -

PYK10 Myrosinase Reveals a Functional Coordination Between Endoplasmic Reticulum Bodies and Glucosinolates in Arabidopsis Thaliana
The Plant Journal (2017) 89, 204–220 doi: 10.1111/tpj.13377 PYK10 myrosinase reveals a functional coordination between endoplasmic reticulum bodies and glucosinolates in Arabidopsis thaliana Ryohei T. Nakano1,2,3, Mariola Pislewska-Bednarek 4, Kenji Yamada5,†, Patrick P. Edger6,‡, Mado Miyahara3,§, Maki Kondo5, Christoph Bottcher€ 7,¶, Masashi Mori8, Mikio Nishimura5, Paul Schulze-Lefert1,2,*, Ikuko Hara-Nishimura3,*,#,k and Paweł Bednarek4,*,# 1Department of Plant Microbe Interactions, Max Planck Institute for Plant Breeding Research, Carl-von-Linne-Weg 10, D-50829 Koln,€ Germany, 2Cluster of Excellence on Plant Sciences (CEPLAS), Max Planck Institute for Plant Breeding Research, Carl-von-Linne-Weg 10, D-50829 Koln,€ Germany, 3Department of Botany, Graduate School of Science, Kyoto University, Sakyo-ku, Kyoto 606-8502, Japan, 4Institute of Bioorganic Chemistry, Polish Academy of Sciences, Noskowskiego 12/14, 61-704 Poznan, Poland, 5Department of Cell Biology, National Institute of Basic Biology, Okazaki 444-8585, Japan, 6Department of Plant and Microbial Biology, University of California, Berkeley, CA 94720, USA, 7Department of Stress and Developmental Biology, Leibniz Institute of Plant Biochemistry, D-06120 Halle (Saale), Germany, and 8Ishikawa Prefectural University, Nonoichi, Ishikawa 834-1213, Japan Received 29 March 2016; revised 30 August 2016; accepted 5 September 2016; published online 19 December 2016. *For correspondence (e-mails [email protected]; [email protected]; [email protected]). #These authors contributed equally to this work. †Present address: Malopolska Centre of Biotechnology, Jagiellonian University, 30-387 Krakow, Poland. ‡Present address: Department of Horticulture, Michigan State University, East Lansing, MI, USA. §Present address: Department of Biological Sciences, Graduate School of Science, The University of Tokyo, Tokyo 113-0033, Japan.