Isolation and Identification of Galangin and Other
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Details of Agreement's Executed in Form – III Sl. No Appl. No. Applicant
Details of Agreement’s Executed in Form – III Sl. Appl. Applicant Name & Contact For Name of the Project / Bioresources Agreement Patent No No. Details m Invention signed on No 1 100 M/s. Scitech Centre, III Invention relates to 1.Glycyrrhiza glabra 12.06.2007 7, Prabhat Nagar, composition and a process 2.Asparagus officinalis Jogeshwari (West), for preparation of a 3.Angelice officinalis Mumbai – 400 012, composition for 4.pimpinella anisum Maharashtra, manufacturing textured 5.Azdiracta indica 6.Acacia India. soluble container using catechu 7.Acorus calamus herbal texturing agent 8.Andrographis paniculata 9.Berberis asiatica 10.Bergenia cordifolia 11.Boerhaavia diffusa 12.Curcuma longa 13.Cuminum cyminum 14.Cinnamomum zilanicum 15.Coriandrum sativum 16.Centella asiatica 17. Clerodendrum paniculatum 18. Dioscorea bulbifera 19.Echinecea purpurea 20.Eclipta alba 21.Foeniculum vulgare 22.Gingiber officinale 23.Gymnema salvastre 24.hemidesmus indicus 25.Hydrastis urge or 26.Nardostachy jatamansi 27.Pueraria tuberose 28.Phyllanthus amarus 29.Picorrhiza kurroa 30.Pluchea lanceolata 31.Ricinus communis 32.Rauvolfia indica 33.Rubia cordifolia 34.Sida cordifolia 35.Saraca asoca 36.Saussurea lappa 37.Terminalia chebula 38.Tinospora cordifolia 39.Tylophora indica 40.Valeriana officinalis 41.Withiana somnifera 2 79 M/s. Vasundhara, III Water detoxication by Coconut Coir 21.06.2007 15, Saheed Nagar, using coconut coir Bhubaneswar – 751 007, Orissa, India. 3 80 M/s. Vasundhara, III Water detoxication by Bacha (Acorus calamus) 21.06.2007 15, Saheed Nagar, using bacha (Acorus rhizomes Bhubaneswar – 751 007, calamus) rhizomes extract Orissa, India. 4 81 M/s. Vasundhara, III Water detoxication by Jamun seed (Syzygium cumini 21.06.2007 15, Saheed Nagar, using Syzygium cumini (L.) Skeels) Bhubaneswar – 751 007, seed extract. -

A Review on Traditional Veterinary Medical Practices in Sri Lanka with Special Reference to Cattle Diseases
Tropical c4gricultural gesearch 8,-- e)aension 13(3): 2010 A REVIEW ON TRADITIONAL VETERINARY MEDICAL PRACTICES IN SRI LANKA WITH SPECIAL REFERENCE TO CATTLE DISEASES ERHSS Ediriweeral, NGAAS Nanayakkara2, OTMRKSB Kalawana2, YKSSL Sugathadasa3 1Department of Nidana Chikithsa, Institute of Indigenous Medicine, University of Colombo, Rajagiriya, Sri Lanka, 2Intern Medical Officer, 3Ayurvedic Physician Accepted: 21st July 2010 ABSTRACT Sri Lanka has well developed systems of traditional veterinary medicine. Unfortunately most of our people today, are unaware of these practices and they generally seek western modern veterinary medic- inal assistance due to this unawareness and also for convenience in their busy lives. Hence, this study was undertaken to collect and preserve the old knowledge and practices. Data were gathered from tra- ditional veterinary physicians in Central and Sabaragamuwa provinces, 011a leaves and text books. These physicians have a knowledge in identifying and treating diseases, preparation of medicines, mode of drug administration, burning of vital points (moxibustion), branding, and blood letting. Specific ap- plications of metaphysical nature such as mystical diagrams and charms, `Yanthra' or 'Kern' are also used. The striking feature of this treatment regimen is the incorporation of both physical (visible) and metaphysical (invisible) methods to treat diseases as preventive and therapeutic measures. The medici- nal preparations consist of herbs, metals, minerals and animal products including milk products, eggs, bones, meat, spider web, anthill mud and human urine. It is also observed that the traditional veteri- nary medicinal practices; especially in treatment of cattle, are very diverse. Key words: Cattle diseases, Traditional veterinary remedies, Sri Lanka INTRODUCTION the knowledge to identify and treat them by using various methods. -

Pharmacological Importance of Kaempferia Galanga (Zingiberaceae): a Mini Review
International Journal of Research in Pharmacy and Pharmaceutical Sciences International Journal of Research in Pharmacy and Pharmaceutical Sciences ISSN: 2455-698X Impact Factor: RJIF 5.22 www.pharmacyjournal.in Volume 3; Issue 3; May 2018; Page No. 32-39 Pharmacological importance of Kaempferia galanga (Zingiberaceae): A mini review Hosne Jahan Shetu1, Kaniz Taskina Trisha2, Shishir Ahmed Sikta3, Raihanatul Anwar4, Sadman Sakib Bin Rashed5, Pritesh Ranjan Dash6* 1, 2, 3 Department of Pharmaceutical Sciences, North South University, Dhaka, Bangladesh 4, 5 Department of Pharmacy, BRAC University, Mohakhali, Dhaka, Bangladesh 6 Department of Pharmacy, Jahangirnagar University, Savar, Dhaka, Bangladesh Abstract Kaempferia galanga L. belonging to the family Zingiberaceae is an endangered medicinal plant with potent medicinal activities. The leaves, rhizome and root tubers of the plant possess a number of medicinal applications. The plant is economically important and is over exploited to the extent that there is always scarcity of propagating material (rhizomes) which is the consumable part too. The present review provides broad information of Kaempferia galanga throwing light on its current status, ethnobotany, phytochemistry and pharmacology. Extracts of Kaempferia galanga have anti-inflammatory, analgesic, anti-diarrheal, anti- bacterial, sedative, cytotoxic, insecticidal and anthelmintic properties which are reported here. Keywords: Kaempferia galanga, zingiberaceae, phytochemistry, pharmacological activity Introduction spite of the variety of useful pharmacological properties it Kaempferia galanga Linn., commonly known as Cekor, possess. Therefore, the importance of the plant K. galanga as Ekangi, Kencur or aromatic ginger is a stem less herb in a medicinal plant is to be documented and presented to the Zingiberaceae family. The plant is native to tropical Asia mass of people. -

Micropropagation-An in Vitro Technique for the Conservation of Alpinia Galanga
Available online a t www.pelagiaresearchlibrary.com Pelagia Research Library Advances in Applied Science Research, 2014, 5(3):259-263 ISSN: 0976-8610 CODEN (USA): AASRFC Micropropagation-an in vitro technique for the conservation of Alpinia galanga Nongmaithem M. Singh 1, Lukram A. Chanu 1, Yendrembam P. Devi 1, Wahengbam R.C. Singh 2 and Heigrujam B. Singh 2 1DBT-Institutional Biotech Hub, Pettigrew College, Ukhrul, Manipur 2DBT- Institutional Biotech Hub, Deptt. of Biotechnology, S.K. Women’s College, Nambol, Manipur _____________________________________________________________________________________________ ABSTRACT This study was conducted to develop an efficient protocol for mass propagation of Alpinia galanga L. Explants from rhizome buds were cultured on Murashige and Skoog (MS) medium supplemented with 6-Benzylaminopurine (BAP) alone (0 to 5 mg/l) or a combination of BAP (0 to 5 mg/l) and indole 3-acetic acid (IAA) (0 to 2 mg/l). MS medium supplemented with a combination of 5.0 mg/l BAP and 2.0 mg/l IAA or 3.0 mg/l BAP and 0.5 mg/l IAA produced the highest mean number of shoots per explant as compared to other concentrations. The best shoot length was obtained on the medium containing 1.0 mg/l of BAP and 2.0 mg/l IAA. Thus, combined effects of BAP and IAA improved significantly the shoot growth and proliferation. MS medium supplemented with a combination of 5.0 mg/l BAP and 2 mg/l IAA gave the highest number of roots. However, longest roots per explant were obtained with 1.0 mg/l BAP alone. -

C-23 Phytochemical of Kaempferia Plant And
Proceeding of International Conference On Research, Implementation And Education Of Mathematics And Sciences 2014, Yogyakarta State University, 18-20 May 2014 C-23 PHYTOCHEMICAL OF KAEMPFERIA PLANT AND BIOPROSPECTING FOR CANCER TREATMENT Sri Atun Chemistry education Faculty of Mathematical and Natural Science, Yogyakarta State University, Jl. Colombo No. 1 Yogyakarta, Indonesia, 55281 e-mail : [email protected] ABSTRACT Kaempferia genus is perennial member of the Zingiberaceae family and is cultivated in Indonesia and other parts of Southeast Asia. Number of studies has been conducted, providing information related to Kaempferia as antioxidant; antimutagenic; and chemopreventive agent. This paper reports some isolated compounds from this plant, biological activity, and bioprospecting for cancer treatment. Keyword: Cancer treatment; Kaempferia; Zingiberaceae INTRODUCTION Kaempferia is a genus, belong to family of Zingiberaceae. This plant grows in Southeast Asia, India, Sri Lanka, Indonesia, and Southem China. Kaempferia genus sinonim with Boesenbergia genus by Baker. This plant has 8 different botanical names which are Boesenbergia cochinchinensis (Gagnep.) Loes., Boesenbergia pandurata (Roxb.) Schltr., Curcuma rotunda L., Gastrochilus panduratus (Roxb.) Ridl., Gastrochilus rotundus (L.) Alston, Kaempferia cochinchinensis Gagnep., Kaempferia ovate Roscoe, Kaempferia galanga, Kaempferia rotunda, and Kaempferia pandurata Roxb nonetheless it is currently known as Boesenbergia rotunda (L.)Mansf (Tan Eng-Chong, et. al, 2012). The plants grown naturally in damp, shaded parts of the lowland or on hill slopes, as scattered plants or thickets. Economically important species among the plant families, the Zingiberaceae, which are perennial rhizomatous herbs, contain volatile oil and other important compounds of enormous medicinal values (Singh C.B., 2013). Phytochemical and biologycal activities of some species of Kaempferia Phytochemical and biologycal some species of plants of the genus Kaempferia reported by many researchers, among others: 1. -
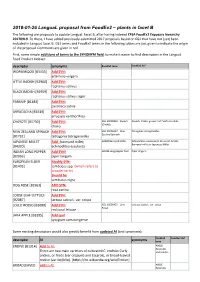
2018-01-26 Langual Proposal from Foodex2 – Plants in Facet B
2018-01-26 LanguaL proposal from FoodEx2 – plants in facet B The following are proposals to update LanguaL Facet B, after having indexed EFSA FoodEx2 Exposure hierarchy 20170919. To these, I have added previously-submitted 2017 proposals based on GS1 that have not (yet) been included in LanguaL facet B. GS1 terms and FoodEx2 terms in the following tables are just given to indicate the origin of the proposal. Comments are given in red. First, some simple additions of terms to the SYNONYM field, to make it easier to find descriptors in the LanguaL Food Product Indexer: descriptor synonyms FoodEx2 term FoodEx2 def WORMWOOD [B3433] Add SYN: artemisia vulgaris LITTLE RADISH [B2960] Add SYN: raphanus sativus BLACK RADISH [B2959] Add SYN: raphanus sativus niger PARSNIP [B1483] Add SYN: pastinaca sativa ARRACACHA [B3439] Add SYN: arracacia xanthorrhiza CHAYOTE [B1730] Add SYN: GS1 10006356 - Squash Squash, Choko, grown from Sechium edule (Choko) choko NEW ZEALAND SPINACH Add SYN: GS1 10006427 - New- Tetragonia tetragonoides Zealand Spinach [B1732] tetragonia tetragonoides JAPANESE MILLET Add : barnyard millet; A000Z Barnyard millet Echinochloa esculenta (A. Braun) H. Scholz, Barnyard millet or Japanese Millet. [B4320] echinochloa esculenta INDIAN LONG PEPPER Add SYN! A019B Long pepper fruit Piper longum [B2956] piper longum EUROPEAN ELDER Modify SYN: [B1403] sambucus spp. (which refers to broader term) Should be sambucus nigra DOG ROSE [B2961] ADD SYN: rosa canina LOOSE LEAF LETTUCE Add SYN: [B2087] lactusa sativa L. var. crispa LOLLO ROSSO [B2088] Add SYN: GS1 10006425 - Lollo Lactuca sativa L. var. crispa Rosso red coral lettuce JAVA APPLE [B3395] Add syn! syzygium samarangense Some existing descriptors would also greatly benefit from updated AI (and synonyms): FoodEx2 FoodEx2 def descriptor AI synonyms term ENDIVE [B1314] Add to AI: A00LD Escaroles There are two main varieties of cultivated C. -

In Vitro Antimicrobial Evaluation of Kaempferia Galanga L. Rhizome Extract Kochuthressia K
AMERICAN JOURNAL BIOTECHNOLOGY AND MOLECULAR SCIENCES ISSN Print: 2159-3698, ISSN Online: 2159-3701, doi:10.5251/ajbms.2012.2.1.1.5 © 2012, ScienceHuβ, http://www.scihub.org/AJBMS In vitro antimicrobial evaluation of Kaempferia galanga L. rhizome extract Kochuthressia K. P.1 S.John Britto2, Jaseentha M.O1 and Rini Raphael3 1Dept of Botany , Carmel College, Mala, Trissur-680732 2The Rapinat Herbarium and Centre for Molecular Systematics St.Joseph’s College (Autonomous), Tiruchirappalli-620 002 3Dept of Zoology, Carmel College, Mala, Trissur-680732 ABSTRACT In the present study, antimicrobial activity of ethanol, methanol, petroleum ether, chloroform and aqueous extracts of Kaempferia galanga rhizome were screened aganist ten human pathogenic bacteria such as Staphylococcus aureus, Streptococcus faecalis, Bacillus cereus, Bacillus subtilis, Enterobacter aerogenes, Salmonella typhi, Escherichia coli, Klebsiella pneumoniae, Pesudomonas aeruginosa and Vibrio cholerae and four fungal species :Aspergillus niger, A . flavus, A.fumigatus and Candida albicans susing disc diffusion assay. All the extracts showed significant antibacterial and antifungal properties. Highest inhibition zone (21.3±0.08) was recorded for ethanolic extract against Staphylococcus aureus. Key words : Rhizome, Kaempferia galanga, antimicrobial activity, disc diffusion assay. INTRODUCTION of medicinal plants are being increasingly reported from different parts of the world (Saxena, 1999). It is Herbal medicines are gaining priorities in treating expected that plant extracts showing target sites various health ailments of diverse origins in man. other than those used by antibiotics will be active Before the inventions of the modern synthetic against drug resistant microbial pathogen medicines, man’s dependence was totally on plants. Traditional systems of plant based products have Kaempferia galanga L. -

Utilization of Garlic and Kaemfiera on the Strength of Fungi Growth in Sardine Fish 'Pedetan' (Sardinella Lemuru)
Utilization of Garlic and Kaemfiera on the Strength of Fungi Growth in Sardine Fish ‘ Pedetan ’ ( Sardinella lemuru ) Ni Made Ayu Suardi Singapurwa 1, I Putu Candra 2 {[email protected] 1, [email protected] 2} Department of Food Science and Technology, Warmadewa University Denpasar-Bali, Indonesia 12 Abstract. Sardine fish ‘ Pedetan ’ is one of the traditional foods in the area of Jembrana Regency, Bali. During the process of storing ‘ Pedetan ’, they are often damaged by fungi that pollute the Pedetan s. This study aims to determine the use of garlic and kaemfiera on the growth of fungi that can contaminate Pedetan . The results showed that the use of garlic and kaemfiera can inhibit fungal growth. The making of sardine fish sprouts using garlic spices can inhibit the growth of fungi with a inhibition zone of 21.15 mm and kaemfiera can inhibit the inhibition zone by 25.45 mm. Garlic and kaemfiera can inhibit fungal growth because they contain bioactive compounds that can be antimicrobial. Keywords: Garlic, kaemfiera, Sardine fish Pedetan 1 Introduction Pedetan is one of the traditional Balinese spicy dried fish food products processed by the community in the Jembrana Regency area of Bali Province. The community processes and extends the shelf life of the sardine by processing it into food products that can be stored longer, which is commonly referred to as Pedetan . Pedetan made from sardine, salt and spices, is dried in the sun for two to three days, then stored at room temperature [1]. Damage to dry fish can occur during storage and during distribution in marketing. -

Insecticidal Activity of Essential Oil Formulas and Their Physiological Effects on Eggplant
Journal Journal of Applied Horticulture, 19(2): 152-158, 2017 Appl Insecticidal activity of essential oil formulas and their physiological effects on eggplant Jarongsak Pumnuan*, Lampan Khurnpoon and Ammorn Insung Faculty of Agricultural Technology, King Mongkut’s Institute of Technology Ladkrabang, Bangkok, 10520, Thailand. *E-mail: [email protected] Abstract This study examined fumigation toxicity of 18 medicinal plant essential oils (EOs) against adult of aphid (Aphid gossypii) and whitefly (Bemisia tabaci). Then, non-target effects of the EO mixtures on physiological changes of eggplant (Solanum melongena) were tested. The insecticidal property and physiological toxicity of the fumigation formulas were also examined and compared to methyl bromide (MB) fumigation. The results showed that the eggplant fumigated with clove (Syzygium aromaticum) and lemon grass (Cymbopogon citratus) EOs mixture at the ratio of 1:3 (Cl1Le3) showed no significant physiological changes when compared to the control treatment. The formula resulted in similarly high mortalities (82-100%) of both insects when compared to MB. However, MB fumigation caused complete senescence appeared before day 3 observations. On the contrary, the eggplant fumigated with Cl1Le3 at 3 µL/L air showed no differences in the physiological changes when compared to the control throughout the 9-day examinations. Key words: Pytotoxicity, methyl bromide, fumigation, clove, lemon grass Introduction application and show considerable commercial significance when used as fumigant in storage containers (Solgi and Ghorbanpour, Vegetables are important export crops of Thailand (OAE, 2013a; 2014). Therefore, many medicinal plant essential oils and their 2013b). However, problems involving insecticide residues and insecticidal organic compounds have been extensively studied in insect contaminations have been threatening the credibility of the recent years. -

Periodic Table of Herbs 'N Spices
Periodic Table of Herbs 'N Spices 11HH 1 H 2 HeHe Element Proton Element Symbol Number Chaste Tree Chile (Vitex agnus-castus) (Capsicum frutescens et al.) Hemptree, Agnus Cayenne pepper, Chili castus, Abraham's balm 118Uuo Red pepper 33LiLi 44 Be 5 B B 66 C 7 N 7N 88O O 99 F 1010 Ne Ne Picture Bear’s Garlic Boldo leaves Ceylon Cinnamon Oregano Lime (Allium ursinum) (Peumus boldus) (Cinnamomum zeylanicum) Nutmeg Origanum vulgare Fenugreek Lemon (Citrus aurantifolia) Ramson, Wild garlic Boldina, Baldina Sri Lanka cinnamon (Myristica fragrans) Oregan, Wild marjoram (Trigonella foenum-graecum) (Citrus limon) 11 Na Na 1212 Mg Mg 1313 Al Al 1414 Si Si 1515 P P 16 S S 1717 Cl Cl 1818 Ar Ar Common Name Scientific Name Nasturtium Alternate name(s) Allspice Sichuan Pepper et al. Grains of Paradise (Tropaeolum majus) (Pimenta dioica) (Zanthoxylum spp.) Perilla (Aframomum melegueta) Common nasturtium, Jamaica pepper, Myrtle Anise pepper, Chinese (Perilla frutescens) Guinea grains, Garden nasturtium, Mugwort pepper, Pimento, pepper, Japanese Beefsteak plant, Chinese Savory Cloves Melegueta pepper, Indian cress, Nasturtium (Artemisia vulgaris) Newspice pepper, et al. Basil, Wild sesame (Satureja hortensis) (Syzygium aromaticum) Alligator pepper 1919 K K 20 Ca Ca 2121 Sc Sc 2222 Ti Ti 23 V V 24 Cr Cr 2525 Mn Mn 2626 Fe Fe 2727 Co Co 2828 Ni Ni 29 Cu Cu 3030 Zn Zn 31 Ga Ga 3232 Ge Ge 3333As As 34 Se Se 3535 Br Br 36 Kr Kr Cassia Paprika Caraway (Cinnamomum cassia) Asafetida Coriander Nigella Cumin Gale Borage Kaffir Lime (Capsicum annuum) (Carum carvi) -

Galangal from Laos to Inhibit Some Foodborne Pathogens, Particularly Escherichia Coli, Salmonella Enterica Serovar
Food and Applied Bioscience Journal, 2018, 6(Special Issue on Food and Applied Bioscience), 218–239 218 Antimicrobial Activities of some Herb and Spices Extracted by Hydrodistillation and Supercritical Fluid Extraction on the Growth of Escherichia coli, Salmonella Typhimurium and Staphylococcus aureus in Microbiological Media Somhak Xainhiaxang1,2, Noppol Leksawasdi1 and Tri Indrarini Wirjantoro1,* Abstract This study investigated the antimicrobial actions of Zanthoxylum limonella, neem leaves, garlic and galangal from Laos to inhibit some foodborne pathogens, particularly Escherichia coli, Salmonella enterica serovar. Typhimurium and Staphylococcus aureus. Herb extracts were obtained by hydrodistillation at 100ºC for 4 h at atmospheric pressure or by supercritical fluid extraction at 45ºC and 17 MPa for 4 h. The antimicrobial activities of the extracts were then studied against three different pathogens on microbiological media using Minimum Inhibitory Concentration (MIC), Minimum Bactericidal Concentration (MBC) and agar disc diffusion assay. The highest yield extract was determined in the Z. limonella extract obtained by hydrodistillation, which was 6.32±0.40%. In the MIC method, the Z. limonella extract from hydrodistillation and galangal extract obtained by supercritical fluid extraction at a concentration of 12.5% could inhibit all of the studied pathogens. However, it was only the Z. limonella extract produced by hydrodistillation that could kill the pathogens at the lowest concentration of 12.5%. Regarding the agar disc diffusion assay, Z. limonella extract from hydrodistillation at 100% concentration could inhibit E. coli for 15.67±1.81 mm, which was not significantly different to that of an antibiotic control of 10 g methicillin (p≥0.05). For S. -

Evaluation of Phytochemicals in Some Indigenous Aromatic Medicinal Plants of North-East India
PharmaTutor PRINT ISSN: 2394-6679 | E-ISSN: 2347-7881 42 Evaluation of phytochemicals in some indigenous aromatic medicinal plants of North-East India *R Sharma, S Sarma Assam Down Town University, Guwahati, Assam, India *[email protected] ABSTRACT Objectives: The aim of the present study was to estimate flavonoid and phenolic content, and to evaluate invitro antioxidant activity of an aqueous extract of Alpinia nigra and Allium tuberosum. Methods: The air dried stem of A. nigra and leaves of A. tuberosum was ground to powder and extracted with water and 95% of ethanol. The extract was screened for phytochemicals, total phenolic content (TPC) and total flavonoid content (TFC) with its potential antioxidant activities using hydrogen peroxide-scavenging assay. Results: Phytochemical test shows that extract contains variety of phytochemicals among which there is a high level of total phenol and flavonoids. The total phenolic content (TPC) of A. nigra and A. tuberosum was 0.450±0.0740 and 1.663±0.296; respectively. The total flavonoid content (TFC) of A. nigra and A. tuberosum was 0.322±0.077 and 0.978±0.119, respectively. The plants possessed potent antioxidant activity when compared with the reference compound ascorbic acid (vitamin C). Conclusions: A. nigra and A. tuberosum may be useful for the preparation of neutraceuticals as potent antioxidant to treat various human diseases and their complications. Keywords: aromatic medicinal plants, North east India, glycosides INTRODUCTION Allium tuberosum belonging to the family North east India comprises seven states commonly Amaryllidaceae is a perennial herb related to onion known as the “Seven Sisters”.