Chapter 4 • Zooplankton
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Appendicularia of CTAW
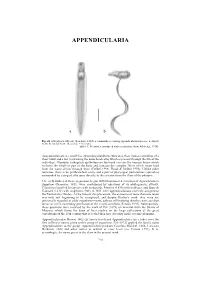
APPENDICULARIA APPENDICULARIA a b Fig. 26. Oikopleura albicans (Leuckart, 1854), a commonly occurring appendicularian species: a, dorsal view; b, lateral view. (Scale bar = 0.1 mm). [after T. Prentiss, reproduced with permission, from Alldredge 1976] Appendicularians are small free swimming planktonic tunicates, their bodies consisting of a short trunk and a tail (containing the notochord cells) which is present through the life of the individual. Glandular (oikoplast) epithelium on the trunk secretes the mucous house which encloses the whole or part of the body and contains the complex filters which strain food from the water driven through them (Deibel 1998; Flood & Deibel 1998). Unlike other tunicates, there is no peribranchial cavity and a pair of pharyngeal perforations (spiracles) surrounded by a ring of cilia open directly to the exterior from the floor of the pharynx. The early studies of these organisms, begun with Chamisso's description of Appendicularia flagellum Chamisso, 1821, were confounded by questions of its phylogenetic affinity. Chamisso classified his species with medusoids, Mertens (1830) with molluscs, and Quoy & Gaimard (1833) with zoophytes. Only in 1851 were appendicularians correctly assigned to the Tunicata by Huxley. At the time of this placement, the existence of more than one taxon was only just beginning to be recognised, and despite Huxley's work, they were not universally regarded as adult organisms—some authors still insisting that they were ascidian larvae or a free swimming generation of the sessile ascidians (Fenaux 1993). Subsequently, these questions were resolved by the work of Fol (1872) on material from the Straits of Messina, which forms the basis of later studies on the large collections of the great expeditions of the 19th century that revealed their true diversity in the oceanic plankton. -

A New Type of Plankton Food Web Functioning in Coastal Waters Revealed by Coupling Monte Carlo Markov Chain Linear Inverse Metho
A new type of plankton food web functioning in coastal waters revealed by coupling Monte Carlo Markov Chain Linear Inverse method and Ecological Network Analysis Marouan Meddeb, Nathalie Niquil, Boutheina Grami, Kaouther Mejri, Matilda Haraldsson, Aurélie Chaalali, Olivier Pringault, Asma Sakka Hlaili To cite this version: Marouan Meddeb, Nathalie Niquil, Boutheina Grami, Kaouther Mejri, Matilda Haraldsson, et al.. A new type of plankton food web functioning in coastal waters revealed by coupling Monte Carlo Markov Chain Linear Inverse method and Ecological Network Analysis. Ecological Indicators, Elsevier, 2019, 104, pp.67-85. 10.1016/j.ecolind.2019.04.077. hal-02146355 HAL Id: hal-02146355 https://hal.archives-ouvertes.fr/hal-02146355 Submitted on 3 Jun 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. 1 A new type of plankton food web functioning in coastal waters revealed by coupling 2 Monte Carlo Markov Chain Linear Inverse method and Ecological Network Analysis 3 4 5 Marouan Meddeba,b*, Nathalie Niquilc, Boutheïna Gramia,d, Kaouther Mejria,b, Matilda 6 Haraldssonc, Aurélie Chaalalic,e,f, Olivier Pringaultg, Asma Sakka Hlailia,b 7 8 aUniversité de Carthage, Faculté des Sciences de Bizerte, Laboratoire de phytoplanctonologie 9 7021 Zarzouna, Bizerte, Tunisie. -

Crustacean Zooplankton in Lake Constance from 1920 to 1995: Response to Eutrophication and Re-Oligotrophication
Arch. Hydrobiol. Spec. Issues Advanc. Limnol. 53, p. 255-274, December 1998 Lake Constance, Characterization of an ecosystem in transition Crustacean zooplankton in Lake Constance from 1920 to 1995: Response to eutrophication and re-oligotrophication Dietmar Straile and Waiter Geller with 9 figures Abstract: During the first three quarters ofthis century, the trophic state ofLake Constance changed from oligotrophic to meso-/eutrophic conditions. The response ofcrustaceans to the eutrophication process is studied by comparing biomasses ofcrustacean zooplankton from recent years, i.e. from 1979-1995, with data from the early 1920s (AUERBACH et a1. 1924, 1926) and the 1950s (MUCKLE & MUCKLE ROTTENGATTER 1976). This comparison revealed a several-fold increase in crustacean biomass. The relative biomass increase was more pronounced from the early 1920s to the 1950s than from the 1950s to the 1980s. Most important changes ofthe species inventory included the invasion of Cyclops vicinus and Daphnia galeata and the extinction of Heterocope borealis and Diaphanosoma brachyurum during the 1950s and early 1960s. All species which did not become extinct increased their biomass during eutrophication. This increase in biomass differed between species and throughout the season which re sulted in changes in relative biomass between species. Daphnids were able to enlarge their seasonal window ofrelative dominance from 3 months during the 1920s (June to August) to 7 months during the 1980s (May to November). On an annual average, this resulted in a shift from a copepod dominated lake (biomass ratio cladocerans/copepods = 0.4 during 1920/24) to a cladoceran dominated lake (biomass ratio cladocerans/copepods = 1.5 during 1979/95). -

Fishery Bulletin/U S Dept of Commerce National Oceanic
NEW RECORDS OF ELLOBIOPSIDAE (PROTISTA (INCERTAE SEDIS» FROM THE NORTH PACIFIC WITH A DESCRIPTION OF THALASSOMYCES ALBATROSSI N.SP., A PARASITE OF THE MYSID STILOMYSIS MAJOR BRUCE L. WINGl ABSTRACT Ten species of ellobiopsids are currently known to occur in the North Pacific Ocean-three on mysids and seven on other crustaceans. Thalassomyces boschmai parasitizes mysids of genera Acanthomysis, Neomysis, and Meterythrops from the coastal waters of Alaska, British Columbia, and Washington. Thalassomyces albatrossi n.sp. is described as a parasite of Stilomysis major from Korea. Thalassomyces fasciatus parasitizes the pelagic mysids Gnathophausia ingens and G. gracilis from Baja California and southern California. Thalassomyces marsupii parasitizes the hyperiid amphipods Parathemisto pacifica and P. libellula and the lysianassid amphipod Cypho caris challengeri in the northeastern Pacific. Thalassomyces fagei parasitizes euphausiids of the genera Euphausia and Thysanoessa in the northeastern Pacific from the southern Chukchi Sea to southern California, and occurs off the coast of Japan in the western Pacific. Thalassomyces capillosus parasitizes the decapod shrimp Pasiphaea pacifica in the northeastern Pacific from Alaska to Oregon, while Thalassomyces californiensis parasitizes Pasiphaea emarginata from central California. An eighth species of Thalassomyces parasitizing pasiphaeid shrimp from Baja California remains undescribed. Ellobiopsis chattoni parasitizes the calanoid copepods Metridia longa and Pseudocalanus minutus in the coastal waters of southeastern Alaska. Ellobiocystis caridarum is found frequently on the mouth parts ofPasiphaea pacifica from southeastern Alaska. An epibiont closely resembling Ellobiocystis caridarum has been found on the benthic gammarid amphipod Rhachotropis helleri from Auke Bay, Alaska. Where sufficient data are available, notes on variability, seasonal occurrence, and effects on the hosts are presented for each species of ellobiopsid. -

Seasonal Dynamics of Meroplankton in a High-Latitude Fjord
1 Seasonal dynamics of meroplankton in a high-latitude fjord 2 3 Helena Kling Michelsen*1, Camilla Svensen1, Marit Reigstad1, Einar Magnus Nilssen1, 4 Torstein Pedersen1 5 6 1Department of Arctic and Marine Biology, UiT the Arctic University of Norway, Tromsø, 7 Norway 8 9 * Corresponding author: [email protected] 10 Faculty of Biosciences, Fisheries and Economics, 11 Department of Arctic and Marine Biology, 12 UiT the Arctic University of Norway, 9037 Tromsø, Norway. 1 13 Abstract 14 Knowledge on the seasonal timing and composition of pelagic larvae of many benthic 15 invertebrates, referred to as meroplankton, is limited for high-latitude fjords and coastal areas. 16 We investigated the seasonal dynamics of meroplankton in the sub-Arctic Porsangerfjord 17 (70˚N), Norway, by examining their seasonal changes in relation to temperature, chlorophyll 18 a and salinity. Samples were collected at two stations between February 2013 and August 19 2014. We identified 41 meroplanktonic taxa from eight phyla. Multivariate analysis indicated 20 different meroplankton compositions in winter, spring, early summer and late summer. More 21 larvae appeared during spring and summer, forming two peaks in meroplankton abundance. 22 The spring peak was dominated by cirripede nauplii, and late summer peak was dominated by 23 bivalve veligers. Moreover, spring meroplankton were the dominant component in the 24 zooplankton community this season. In winter, low abundances and few meroplanktonic taxa 25 were observed. Timing for a majority of meroplankton correlated with primary production 26 and temperature. The presence of meroplankton in the water column through the whole year 27 and at times dominant in the zooplankton community, suggests that they, in addition to being 28 important for benthic recruitment, may play a role in the pelagic ecosystem as grazers on 29 phytoplankton and as prey for other organisms. -

Chemists with No Backbones
Medicines from the Deep Sea: Exploration of the Gulf of Mexico Chemists with No Backbones FOCUS TEACHING TIME Benthic invertebrates that produce pharmacologi- One or two 45-minute class periods, plus time for cally-active substances student research GRADE LEVEL SEATING ARRANGEMENT 5-6 (Life Science) Classroom style, or groups of 2-3 students FOCUS QUESTION MAXIMUM NUMBER OF STUDENTS What groups of marine organisms produce sub- 30 stances that may be helpful in treating human dis- eases? KEY WORDS Cardiovascular disease LEARNING OBJECTIVES Cancer Students will be able to identify at least three Arthritis groups of benthic invertebrates that are known to Natural products produce pharmacologically-active compounds. Sponge Tunicate Students will be able to describe why pharmaco- Ascidian logically-active compounds derived from benthic Bryozoan invertebrates may be important in treating human Octocorals diseases. BACKGROUND INFORMATION Students will be able to infer why sessile marine Despite the many advances of modern medicine, invertebrates appear to be promising sources of disease is still the leading cause of death in the new drugs. United States. Cardiovascular disease and cancer together account for more than 1.5 million deaths MATERIALS annually (40% and 25% of all deaths, respectively). Marker board, blackboard, or overhead projector In addition, one in six Americans have some form with transparencies for group discussions of arthritis, and hospitalized patients are increas- ingly threatened by infections that are resistant to AUDIO/VISUAL MATERIALS conventional antibiotics. The cost of these diseases None is staggering: $285 billion per year for cardiovas- cular disease; $107 billion per year for cancer; $65 billion per year for arthritis. -

Full Text in Pdf Format
MARINE ECOLOGY PROGRESS SERIES Vol. 166: 301-306, 1998 Published May 28 Mar Ecol Prog Ser l NOTE Diel vertical movement by mesograzers on seaweeds Cary N. Rogers*, Jane E. Williamson, David G. Carson, Peter D. Steinberg School of Biological Science. University of New South Wales. Sydney 2052. Australia ABSTRACT- Diel vertical movement is well documented for nutrients across trophic levels in such systems (Kitting many zooplankton. The ecology of small benthic herbivores et al. 1984, Longhurst & Harrison 1989). which use seaweeds as food and habitat, known as 'meso- Arguably, the dominant model to explain die1 verti- grazers', is similar in some regards to zooplankton, and we hypothesised that mesograzers might also exhlbit diel pat- cal movement by zooplankton is avoidance of visually terns of movement on host algae. We studied 3 non-swim- feeding predators in surface waters during the day ming species of mesograzer, the sea hare Aplysia parvula, the (Zaret & Suffern 1976, Stich & Lampert 1981, Bollens & sea urchin Holopneustes purpurascens, and the prosobranch Frost 1989).This model is supported by studies of both mollusc Phasianotrochus eximius. All exhibited diel move- & ment on host algae. This behaviour occurred on different host demersal and pelagic zooplankton (Robertson algae, despite variation in algal morphology and other char- Howard 1978, Alldredge & King 1985, Ohman 1990, acters. Possible factors causing diel movement by mesograz- Osgood & Frost 1994), although the impact of preda- ers include predation, nutritional gain, avoidance of photo- tion on zooplankton vanes with nntogenetic stage, damage, micro-environmental vanation near host algae, and food availability, and predator evasion or defence reproductive strategies. -
Diel and Ontogenetic Variations in Vertical Distributions of Large Grazing Copepods During the Spring Phytoplankton Title Bloom in the Oyashio Region
Diel and ontogenetic variations in vertical distributions of large grazing copepods during the spring phytoplankton Title bloom in the Oyashio region Author(s) Yamaguchi, Atsushi; Onishi, Yuka; Kawai, Momoka; Omata, Aya; Kaneda, Mariko; Ikeda, Tsutomu Deep Sea Research Part II: Topical Studies in Oceanography, 57(17-18), 1691-1702 Citation https://doi.org/10.1016/j.dsr2.2010.03.013 Issue Date 2010-09 Doc URL http://hdl.handle.net/2115/43807 Type article (author version) File Information DSR2-57-17-18_1691-1702.pdf Instructions for use Hokkaido University Collection of Scholarly and Academic Papers : HUSCAP 1 Diel and ontogenetic variations in vertical distributions of large grazing copepods 2 during the spring phytoplankton bloom in the Oyashio region 3 Atsushi Yamaguchi*, Yuka Onishi, Momoka Kawai, Aya Omata, Mariko Kaneda, 4 Tsutomu Ikeda 5 Graduate School of Fisheries Sciences, Hokkaido University, 3-1-1 Minato, Hakodate, 6 Hokkaido 041-8611, Japan 7 *Corresponding author. 8 Tel: +81-138-40-5543 9 Fax: +81-138-40-5542 10 E-mail: [email protected] 1 11 Abstract 12 Short-term changes in vertical distributions of copepods during the spring 13 phytoplankton bloom were analyzed based on day and night vertically stratified 14 sampling (9 strata between 0-1000 m) with a fine-mesh (60 µm) VMPS in the Oyashio 15 region on five occasions: 8 March, 5, 11, 23 and 29 April 2007. Responses to the 16 bloom were varied and species-specific. Eucalanus bungii C3-C6 were resting around 17 400 m on 8 March. They had aroused from rest by 5 April, more completely for C6F 18 than for C3-C4. -

Zooplankton Chapters 6-8 in Miller for More Details 1. Crustaceans- Include Shrimp, Copepods, Euphausiids ("Krill") 2
Ocean Processes and Ecology Spring 2004 Zooplankton Chapters 6-8 in Miller for more details I. Major Groups Heterotrophs— consume organic matter rather than manufacturing it, as do autotrophs. Zooplankton can be: herbivores carnivores (several levels) detritus feeders omnivores Zooplankton, in addition to being much smaller than familiar land animals, have shorter generation times and grow more rapidly (in terms of % of body wt / day). 1. Crustaceans- include shrimp, copepods, euphausiids ("krill") Characteristics: Copepods, euphausiids and shrimp superficially resemble one another. All have: • exoskeletons of chitin • jointed appendages • 2 pair of antennae • complex body structure, with well developed internal organs and sensory organs Habitats: Ubiquitous. • Euphausiids predominate in the Antarctic Ocean, but are common in most temperate and polar oceans. • Copepods are found everywhere, but are less important in low-productivity areas of the ocean - the "central ocean gyres". They are found at all depths but are more abundant near the surface. Role in food webs: • Euphausiids and copepods are filter-feeders. Copepods are usually herbivores, while the larger euphausiids consume both phytoplankton and other zooplankton. • Shrimp are usually carnivores or scavengers. 2. Chaetognaths - ("Arrow worms") Characteristics: • 2-3 cm long • wormlike, but non-segmented • no appendages (legs or antennae) • complex body structure with internal organs Habitat: Ubiquitous Ocean Processes and Ecology Spring 2004 Role in food web: Carnivore feeding on small zooplankton such as copepods. 3. Protozoan - Include foraminifera, radiolarians, tintinnids and "microflagellates" ca. 0.002 mm Characteristics: • Single-celled animals. • Forams have calcareous shell • Radiolarians have siliceous shell. • Both Forams and Radiolarians have spines. Habitat: Ubiquitous • Radiolarians are especially abundant in the Pacific equatorial upwelling region. -

Solomon Islands Marine Life Information on Biology and Management of Marine Resources
Solomon Islands Marine Life Information on biology and management of marine resources Simon Albert Ian Tibbetts, James Udy Solomon Islands Marine Life Introduction . 1 Marine life . .3 . Marine plants ................................................................................... 4 Thank you to the many people that have contributed to this book and motivated its production. It Seagrass . 5 is a collaborative effort drawing on the experience and knowledge of many individuals. This book Marine algae . .7 was completed as part of a project funded by the John D and Catherine T MacArthur Foundation Mangroves . 10 in Marovo Lagoon from 2004 to 2013 with additional support through an AusAID funded community based adaptation project led by The Nature Conservancy. Marine invertebrates ....................................................................... 13 Corals . 18 Photographs: Simon Albert, Fred Olivier, Chris Roelfsema, Anthony Plummer (www.anthonyplummer. Bêche-de-mer . 21 com), Grant Kelly, Norm Duke, Corey Howell, Morgan Jimuru, Kate Moore, Joelle Albert, John Read, Katherine Moseby, Lisa Choquette, Simon Foale, Uepi Island Resort and Nate Henry. Crown of thorns starfish . 24 Cover art: Steven Daefoni (artist), funded by GEF/IWP Fish ............................................................................................ 26 Cover photos: Anthony Plummer (www.anthonyplummer.com) and Fred Olivier (far right). Turtles ........................................................................................... 30 Text: Simon Albert, -

Biological Oceanography - Legendre, Louis and Rassoulzadegan, Fereidoun
OCEANOGRAPHY – Vol.II - Biological Oceanography - Legendre, Louis and Rassoulzadegan, Fereidoun BIOLOGICAL OCEANOGRAPHY Legendre, Louis and Rassoulzadegan, Fereidoun Laboratoire d'Océanographie de Villefranche, France. Keywords: Algae, allochthonous nutrient, aphotic zone, autochthonous nutrient, Auxotrophs, bacteria, bacterioplankton, benthos, carbon dioxide, carnivory, chelator, chemoautotrophs, ciliates, coastal eutrophication, coccolithophores, convection, crustaceans, cyanobacteria, detritus, diatoms, dinoflagellates, disphotic zone, dissolved organic carbon (DOC), dissolved organic matter (DOM), ecosystem, eukaryotes, euphotic zone, eutrophic, excretion, exoenzymes, exudation, fecal pellet, femtoplankton, fish, fish lavae, flagellates, food web, foraminifers, fungi, harmful algal blooms (HABs), herbivorous food web, herbivory, heterotrophs, holoplankton, ichthyoplankton, irradiance, labile, large planktonic microphages, lysis, macroplankton, marine snow, megaplankton, meroplankton, mesoplankton, metazoan, metazooplankton, microbial food web, microbial loop, microheterotrophs, microplankton, mixotrophs, mollusks, multivorous food web, mutualism, mycoplankton, nanoplankton, nekton, net community production (NCP), neuston, new production, nutrient limitation, nutrient (macro-, micro-, inorganic, organic), oligotrophic, omnivory, osmotrophs, particulate organic carbon (POC), particulate organic matter (POM), pelagic, phagocytosis, phagotrophs, photoautotorphs, photosynthesis, phytoplankton, phytoplankton bloom, picoplankton, plankton, -

Zooplankton Community Response to Seasonal Hypoxia: a Test of Three Hypotheses
diversity Article Zooplankton Community Response to Seasonal Hypoxia: A Test of Three Hypotheses Julie E. Keister *, Amanda K. Winans and BethElLee Herrmann School of Oceanography, University of Washington, Box 357940, Seattle, WA 98195, USA; [email protected] (A.K.W.); [email protected] (B.H.) * Correspondence: [email protected] Received: 7 November 2019; Accepted: 28 December 2019; Published: 1 January 2020 Abstract: Several hypotheses of how zooplankton communities respond to coastal hypoxia have been put forward in the literature over the past few decades. We explored three of those that are focused on how zooplankton composition or biomass is affected by seasonal hypoxia using data collected over two summers in Hood Canal, a seasonally-hypoxic sub-basin of Puget Sound, Washington. We conducted hydrographic profiles and zooplankton net tows at four stations, from a region in the south that annually experiences moderate hypoxia to a region in the north where oxygen remains above hypoxic levels. The specific hypotheses tested were that low oxygen leads to: (1) increased dominance of gelatinous relative to crustacean zooplankton, (2) increased dominance of cyclopoid copepods relative to calanoid copepods, and (3) overall decreased zooplankton abundance and biomass at hypoxic sites compared to where oxygen levels are high. Additionally, we examined whether the temporal stability of community structure was decreased by hypoxia. We found evidence of a shift toward more gelatinous zooplankton and lower total zooplankton abundance and biomass at hypoxic sites, but no clear increase in the dominance of cyclopoid relative to calanoid copepods. We also found the lowest variance in community structure at the most hypoxic site, in contrast to our prediction.