BARK and ITS POSSIBLE USES Summary
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bark Anatomy and Cell Size Variation in Quercus Faginea
Turkish Journal of Botany Turk J Bot (2013) 37: 561-570 http://journals.tubitak.gov.tr/botany/ © TÜBİTAK Research Article doi:10.3906/bot-1201-54 Bark anatomy and cell size variation in Quercus faginea 1,2, 2 2 2 Teresa QUILHÓ *, Vicelina SOUSA , Fatima TAVARES , Helena PEREIRA 1 Centre of Forests and Forest Products, Tropical Research Institute, Tapada da Ajuda, 1347-017 Lisbon, Portugal 2 Centre of Forestry Research, School of Agronomy, Technical University of Lisbon, Tapada da Ajuda, 1349-017 Lisbon, Portugal Received: 30.01.2012 Accepted: 27.09.2012 Published Online: 15.05.2013 Printed: 30.05.2013 Abstract: The bark structure of Quercus faginea Lam. in trees 30–60 years old grown in Portugal is described. The rhytidome consists of 3–5 sequential periderms alternating with secondary phloem. The phellem is composed of 2–5 layers of cells with thin suberised walls and narrow (1–3 seriate) tangential band of lignified thick-walled cells. The phelloderm is thin (2–3 seriate). Secondary phloem is formed by a few tangential bands of fibres alternating with bands of sieve elements and axial parenchyma. Formation of conspicuous sclereids and the dilatation growth (proliferation and enlargement of parenchyma cells) affect the bark structure. Fused phloem rays give rise to broad rays. Crystals and druses were mostly seen in dilated axial parenchyma cells. Bark thickness, sieve tube element length, and secondary phloem fibre wall thickness decreased with tree height. The sieve tube width did not follow any regular trend. In general, the fibre length had a small increase toward breast height, followed by a decrease towards the top. -

ADVISORY BOARDS Each Issue of Herbaigram Is Peer Reviewed by Various Members of Our Advisory Boards Prior to Publication
ADVISORY BOARDS Each issue of HerbaiGram is peer reviewed by various members of our Advisory Boards prior to publication . American Botanical Council Herb Research Dennis V. C. Awang, Ph.D., F.C.I.C., MediPiont Natural Gail B. Mahadr, Ph.D., Research Assistant Professor, Products Consul~ng Services, Ottowa, Ontario, Conodo Deportment o Medical Chemistry &Pharmacognosy, College of Foundation Pharmacy, University of Illinois, Chicago, Illinois Manuel F. Balandrin, R.Ph., Ph.D., Research Scien~st, NPS Rob McCaleb, President Pharmaceuticals, Salt LakeCity , Utah Robin J. Maries, Ph.D., Associate Professor of Botany, Brandon University, Brandon, Manitoba, Conodo Mi(hael J. Balidt, Ph.D., Director of the lns~tute of Econom ic Glenn Appelt, Ph.D., R.Ph., Author and Profess or Botany, the New York Botanical Gorden, Bronx, New York Dennis J. M(Kenna, Ph.D., Consulting Ethnophormocologist, Emeritus, University of Colorado, and with Boulder Beach Joseph M. Betz, Ph.D., Research Chemist, Center for Food Minneapolis, Minnesota Consulting Group Safety and Applied Nutri~on, Division of Natural Products, Food Daniel E. Moerman, Ph.D., William E. Stirton Professor of John A. Beutler, Ph.D., Natural Products Chemist, and Drug Administro~on , Washington, D.C. Anthropology, University of Michigon/ Deorbom, Dearborn, Michigan Notional Cancer Institute Donald J. Brown, N.D., Director, Natural Products Research Consultants; Faculty, Bastyr University, Seattle, Washington Samuel W. Page, Ph.D., Director, Division of Natural Products, Robert A. Bye, Jr., Ph.D., Professor of Ethnobotony, Notional University of Mexico Thomas J. Carlson, M.S., M.D., Senior Director, Center for Food Safety and Applied Nutri~on , Food and Drug Administro~on , Washington, D.C. -

Dendrometric Analysis of Early Development of Eucalyptus Urophylla X Eucalyptus Grandis with Gypsum Use Under Subtropical Conditions
Floresta e Ambiente 2020; 27(1): e20190095 https://doi.org/10.1590/2179-8087.009519 ISSN 2179-8087 (online) Original Article Silviculture Dendrometric Analysis of Early Development of Eucalyptus urophylla x Eucalyptus grandis with Gypsum use Under Subtropical Conditions Carla Fernanda Ferreira1, Marcos Vinicius Martins Bassaco2 , Milena Pereira3, Volnei Pauletti3, Stephen Arthur Prior4 , Antonio Carlos Vargas Motta3 1Departamento de Agronomia, Centro de Ensino Superior dos Campos Gerais – CESCAGE, Ponta Grossa/PR, Brasil 2 Departamento de Engenharia Florestal, Faculdades FATI-FAJAR, Jaguariaíva/PR, Brasil 3Departamento de Solos e Engenharia Agrícola, Universidade Federal do Paraná – UFPR, Curitiba/PR, Brasil 4ARS National Soil Dynamics Laboratory, Auburn, USA ABSTRACT Gypsum can be used as a source for calcium (Ca) and sulphurum (S) for plants, as well as an acid, that is, a natural soil conditioner. Aiming to determine the influence of gypsum on the development of Eucalyptus urograndis in Brazil, an experiment was conducted at two locations in Paraná State. Experiments were conducted with rates of 0, 0.3, 0.6, 1.2, 2.4, 4.8 and 9.6 Mg ha-1 to verify the method of broadcast planting in a randomized block design with four repetitions. Diameter and height of plants were measured every six months and volume was determined after 36 months. There was a difference in Eucalyptus growth between the two areas, possibly related to differences in planting season and climate. Gypsum did not influence on the dendrometric growth of Eucalyptus trees. The lack of a response to gypsum, as a source of Ca, S and soil conditioner, was discussed based on soil type, Eucalyptus tolerance to soil acidity, and climatic conditions in the period evaluated. -

Opens in a New Windowguidelines for Pesticide Registration
GUIDELINES FOR PESTICIDE REGISTRATION CONTROL OF PLANTS ACT, CHAPTER 57A. Control of Plants (Registration of Pesticides) Rules. General Information A. All agricultural pesticide1 products used in the cultivation of plants intended for sale must be registered with the Singapore Food Agency (SFA).These include pesticide-containing biostimulant/fertilizer/soil conditioner. Biostimulant/fertilizer/soil conditioner which does not contain pesticide, or pesticide products for other purposes, including export, and industrial, public hygiene, landscaping and household uses, do not require registration with SFA. Pesticides used for the formulation of pesticide products also do not require registration with SFA. B. Any person who manufactures, imports, distributes, supplies or sells any pesticide products and who is conducting business in Singapore which is registered under the Business Names Registration Act 2014, or any company incorporated under the Companies Act, may apply for the registration of pesticide products for use in the agricultural farms in Singapore. C. Before applying for registration of a pesticide product with SFA, applicants are required to check whether the import of the pesticide for local use is allowed by the following agencies: i) Pollution Control Department (PCD) of the National Environment Agency (NEA) for import, export and sale of chemical pesticides that are listed as hazardous substances ii) Plant Health Services of the National Parks Board (NParks) for import of biological pesticides and organic fertilizers [Please check with NEA if you intend to register pesticides for vector control purposes.] D. If applicants are dealing with pesticides that are listed in the Environmental Protection and Management Act (EPMA), a copy of the Hazardous Substances Licence issued by the PCD/NEA must accompany the application. -

The Effect of Coconut Extract on Callus Growth and Ultrasound Waves On
Folia Forestalia Polonica, Series A – Forestry, 2018, Vol. 60 (4), 261–268 METHODOLOGICAL ARTICLE DOI: 10.2478/ffp-2018-0027 The effect of coconut extract on callus growth and ultrasound waves on production of betulin and betulinic acid in in-vitro culture conditions of Betula pendula Roth species Vahide Payamnoor1 , Razieh Jafari Hajati2, Negar Khodadai1 1 Gorgan University of Agricultural Sciences and Natural Resources, Faculty of Forest Sciences, Gorgan, Iran, phone: 0098-9113735812, e-mail: [email protected] 2 Shahed University, Traditional Clinical Trial Research Center, Tehran, Iran AbstrAct To determine the effect of coconut extract on callogenesis of Betula pendula, Roth stem barks were cultured in NT (Nagata and Takebe) basic culture media in two individual experiments: i) cultivation explant in different treat- ments of coconut extracts combined with 1 mg l-1 2, 4-D (2, 4-Dichlorophenoxyacetic acid) and ii) callogenesis in NT media containing 1.5 mg l-1 2,4-D and 0.5 mg l-1 BAP (6-Benzylaminopurine) and then cultivation under the first experiment treatments. The first experiment demonstrated that not all concentrations of coconut extracts lead to callus induction individually, but callus induction increased 84% in a culture containing 5% coconut extract plus 1 mg l-1 2, 4-D. Based on the results of the second experiment, this treatment also significantly increased the wet and dry weights of the produced calluses. The possibility of increasing the betulinic acid and betulin by ultrasound was also studied. Samples cultivated in the selected culture medium were exposed to ultrasound waves in two forms of 1) one exposure and 2) twice exposure (repetition with 24 hr interval) in steps of 20, 60, 100, and 160 sec, and one treatment as the control. -

Code of Practice for Wood Processing Facilities (Sawmills & Lumberyards)
CODE OF PRACTICE FOR WOOD PROCESSING FACILITIES (SAWMILLS & LUMBERYARDS) Version 2 January 2012 Guyana Forestry Commission Table of Contents FOREWORD ................................................................................................................................................... 7 1.0 INTRODUCTION ...................................................................................................................................... 8 1.1 Wood Processing................................................................................................................................. 8 1.2 Development of the Code ................................................................................................................... 9 1.3 Scope of the Code ............................................................................................................................... 9 1.4 Objectives of the Code ...................................................................................................................... 10 1.5 Implementation of the Code ............................................................................................................. 10 2.0 PRE-SAWMILLING RECOMMENDATIONS. ............................................................................................. 11 2.1 Market Requirements ....................................................................................................................... 11 2.1.1 General .......................................................................................................................................... -

Regulation on Forest Products Processing and Marketing
Forestry Development Authority Regulation No. 112-08 Regulation on the Forest Products Processing and Marketing WHEREAS, the National Forestry Reform Law of 2006 establishes a transparent framework, for the use, management and protection of forest resources that balances the commercial, community, and conservation priorities of the Republic; and WHEREAS, the economic potential of processed wood products has not been appreciated, there has been too much exportation of crude round logs with evidence of low percentage of primary processed wood for both domestic and export market, no value-added wood product, low recovery rate of wood processing industry, problem of waste disposal; and WHEREAS, industrialization of the wood industry in general and the processing of wood into secondary/value-added products in particular are essential elements for the realization of the potential sustainable utilization of Liberia‘s forest resources; and WHEREAS, sustainable utilization of forest resources concept is not only to maintain the value of forest resources, but rather it also has a huge potential for creating employment, income and wealth for the Liberia populations; and WHEREAS, local small-scale wood enterprises are not fully capacitated and unregulated which in essence has resulted into unsustainable use and marketing of wood products, wastage of wood products, constant exposure of wood products to deteriorating agents; and WHEREAS, the lack of standard units of measurement for Liberia domestic wood market and unregulated import of wood products, -

Tree Identification: from Bark and Leaves Or Needles
Tree Identification: from bark and leaves or needles A walk in the woods can be a lot of fun, especially if you bring your kids. How do you get them to come along with you? Tell them this. “Look at the bark on the trees. Can you can find any that look like burnt potato chips, warts, cat scratches, camouflage pants or rippling muscles?” Believe it or not, these are descriptions of different kinds of tree bark. Tree ID from bark:______________________________________________________ Black cherry: The bark looks like burnt potato chips. Hackberry: The bark is bumpy and warty. Ironwood: The bark has long thin strips. With a little imagination, an ironwood can look like it is used as a scratching post by cats. They can also be easily spotted in winter because their light brown dead leaves hang on well past the first snow. Sycamore: A tree with bark that looks like camouflaged pants. The largest tree in Illinois is a sycamore, a majestic 115-foot tree near Springfield. Musclewood: a tree with smooth gray bark covering a trunk with ridges that look like they are rippling muscles. Shagbark hickory: Long strips of shaggy bark peeling at both ends. Cottonwood: Has heavy ridges that make it look like Paul Bunyan’s corduroy pants. Bur oak: Thick and gnarly bark has deep craggy furrows. The corky bark allows it to easily withstand hot forest fires. Tree ID from leaves or needles:________________________________________ Willow Oak: Has elongated leaves similar to those of a willow tree. Red pine: Red has 3 letters; a red pine has groupings of 2-3 prickly needles. -

Soil Conditioner Recovery Product Construction Amendment
Guaranteed Analysis DIRECTIONS FOR USE Total Nitrogen (N) ...........................1.0% 0.5% Water Soluble Nitrogen Aerification Available Phosphate (P2O5) ................0% Renovate Plus is a recommended aerification Soluble Potash (K2O) ......................1.0% product. A wide range of application rates can Calcium (Ca) .................................5.0% be made depending on the desired outcome. Iron (Fe) .........................................2.0% Recommended rates are 10 to 25 pounds of Renovate P Renovate Plus Renovate product per 1,000 sq. ft. dragged into aeration Ingredients: CARBON BASED FERTILITY holes followed by watering the soil surface. L Compost, kelp, bone meal, greensand, rock Sod & Seed Establishment U phosphate, calcium sulfate, zeolites, humates. Apply Renovate Plus directly to the soil surface and scarify into the top inch or two of soil. Information regarding the contents and levels S Recommended rates are 10 to 25 pounds of of metals in this product is available on the e way s product per 1000 sq. ft. Internet at: g th ince http://www.aapfco.org/metals.htm din 19 Sand Construction Amendment ea 88 Up to 800 pounds of Renovate Plus per 1000 L sq. ft. worked into the top 6 to 8 inches of sand is recommended for sand root zone construc- tion. A complete chemistry soil testing protocol should accompany all construction programs including a particle size analysis. Lawn Care and Turf Maintenance Apply 10 to 25 pounds of product per 1000 sq. ft. as a top dressing ideally accompanied with TM surface spiking. On the weakest areas use the higher rates and water in thoroughly. Ornamental Planting Renovate Plus is a recommended planting amendment for woody ornamentals, perennials and annuals. -

Urban Wood and Traditional Wood
FNR-490-W AGEXTENSIONRICULTURE Authors Daniel L. Cassens, Professor of Wood Products and Extension Urban Wood and Traditional Wood: Specialist, Purdue University Edith Makra, Chairman, A Comparison of Properties and Uses Illinois Emerald Ash Borer Wood Utilization Team Trees are cultivated in public and private wood. This publication describes some key landscapes in and around cities and differences between wood products from towns. They are grown for the tremendous traditional forests and those available from contributions they make both to the urban forests. Because the urban wood environment and the quality of people’s lives. industry is emerging and the knowledge In this urban forest, trees must be removed base is still sparse, conclusions drawn in the when they die or for reasons of health, safety, publication are based on knowledge of urban or necessary changes in the landscape. The forestry and of the traditional forest products wood from these felled landscape trees industry. could potentially be salvaged and used to manufacture wood products, but not in the Forest Management same way as forest-grown trees. Traditional forests are either managed specifically to produce commodity wood or The traditional forest products industry is to meet stewardship objectives compatible based on forest-grown trees; its markets with responsible harvesting, such as for and systems don’t readily adapt to this new watershed and wildlife. Harvesting is source of urban wood. The urban forest typically done in accordance with long-term grows different trees in a different manner forest management plans that sustain forest than the traditional forest, and the wood health and suit landowner objectives. -

Wood As a Sustainable Building Material Robert H
CHAPTER 1 Wood as a Sustainable Building Material Robert H. Falk, Research General Engineer Few building materials possess the environmental benefits of wood. It is not only our most widely used building mate- Contents rial but also one with characteristics that make it suitable Wood as a Green Building Material 1–1 for a wide range of applications. As described in the many Embodied Energy 1–1 chapters of this handbook, efficient, durable, and useful wood products produced from trees can range from a mini- Carbon Impact 1–2 mally processed log at a log-home building site to a highly Sustainability 1–3 processed and highly engineered wood composite manufac- tured in a large production facility. Forest Certification Programs 1–3 As with any resource, we want to ensure that our raw ma- Forest Stewardship Council (FSC) 1–4 terials are produced and used in a sustainable fashion. One Sustainable Forestry Initiative (SFI) 1–4 of the greatest attributes of wood is that it is a renewable resource. If sustainable forest management and harvesting American Tree Farm System (ATFS) 1–4 practices are followed, our wood resource will be available Canadian Standards Association (CSA) 1–5 indefinitely. Programme for the Endorsement of Forest Certification (PEFC) Schemes 1–5 Wood as a Green Building Material Over the past decade, the concept of green building1 has Additional Information 1–5 become more mainstream and the public is becoming aware Literature Cited 1–5 of the potential environmental benefits of this alternative to conventional construction. Much of the focus of green building is on reducing a building’s energy consumption (such as better insulation, more efficient appliances and heating, ventilation, and air-conditioning (HVAC) systems) and reducing negative human health impacts (such as con- trolled ventilation and humidity to reduce mold growth). -
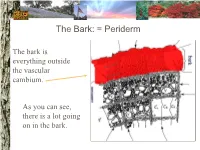
The Bark: = Periderm
The Bark: = Periderm The bark is everything outside the vascular cambium. As you can see, there is a lot going on in the bark. The Bark: periderm: phellogen (cork cambium): The phellogen is the region of cell division that forms the periderm tissues. Phellogen development influences bark appearance. The Bark: periderm: phellem (cork): Phellem replaces the epidermis as the tree increases in girth. Photosynthesis can take place in some trees both through the phellem and in fissures. The Bark: periderm: phelloderm: Phelloderm is active parenchyma tissue. Parenchyma cells can be used for storage, photosynthesis, defense, and even cell division! The Bark: phloem: Phloem tissue makes up the inner bark. However, it is vascular tissue formed from the vascular cambium. The Bark: phloem: sieve tube elements: Sieve tube elements actively transport photosynthates down the stem. Conifers have sieve cells instead. The cambium: The cambium is the primary meristem producing radial growth. It forms the phloem & xylem. The Xylem (wood): The xylem includes everything inside the vascular cambium. The Xylem: a growth increment (ring): The rings seen in many trees represent one growth increment. Growth rings provide the texture seen in wood. The Xylem: vessel elements: Hardwood species have vessel elements in addition to trachieds. Notice their location in the growth rings of this tree The Xylem: fibers: Fibers are cells with heavily lignified walls making them stiff. Many fibers in sapwood are alive at maturity and can be used for storage. The Xylem: axial parenchyma: Axial parenchyma is living tissue! Remember that parenchyma cells can be used for storage and cell division.