A High Resolution Atlas of Gene Expression in the Domestic Sheep
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Generation and Characterization of a Novel Mouse Line, Keratocan-Rtta (Kerart), for Corneal Stroma and Tendon Research
Cornea Generation and Characterization of a Novel Mouse Line, Keratocan-rtTA (KeraRT), for Corneal Stroma and Tendon Research Yujin Zhang,1 Winston W.-Y. Kao,2 Yasuhito Hayashi,3 Lingling Zhang,1 Mindy Call,2 Fei Dong,2 Yong Yuan,2 Jianhua Zhang,2 Yen-Chiao Wang,1 Okada Yuka,1,4 Atsushi Shiraishi,3 and Chia-Yang Liu1 1School of Optometry, Indiana University, Bloomington, Indiana, United States 2Edith J. Crawley Vision Research Center/Department of Ophthalmology, University of Cincinnati College of Medicine, Cincinnati, Ohio, United States 3Department of Ophthalmology, School of Medicine, Ehime University, Ehime, Japan 4Department of Ophthalmology, School of Medicine, Wakayama Medical University, Wakayama, Japan RT Correspondence: Yujin Zhang, Indi- PURPOSE. We created a novel inducible mouse line Keratocan-rtTA (Kera ) that allows ana University School of Optometry, specific genetic modification in corneal keratocytes and tenocytes during development and in 800 Atwater Avenue, Bloomington, adults. IN 47405, USA; [email protected]. METHODS. A gene-targeting vector (Kera- IRES2-rtTA3) was constructed and inserted right after Chia-Yang Liu, Indiana University the termination codon of the mouse Kera allele via gene targeting techniques. The resulting RT RT School of Optometry, 800 Atwater Kera mouse was crossed to tet-O-Hist1H2B-EGFP (TH2B-EGFP) to obtain Kera /TH2B-EGFP Avenue, Bloomington, IN 47405, compound transgenic mice, in which cells expressing Kera are labeled with green USA; fluorescence protein (GFP) by doxycycline (Dox) induction. The expression patterns of [email protected]. RT RT GFP and endogenous Kera were examined in Kera /TH2B-EGFP. Moreover, Kera was bred Submitted: July 21, 2017 with tet-O-TGF-a to generate a double transgenic mouse, KeraRT/tet-O-TGF-a, to overexpress Accepted: August 16, 2017 TGF-a in corneal keratocytes upon Dox induction. -

Mitochondrial Dynamics During Spermatogenesis Grigor Varuzhanyan and David C
© 2020. Published by The Company of Biologists Ltd | Journal of Cell Science (2020) 133, jcs235937. doi:10.1242/jcs.235937 REVIEW SUBJECT COLLECTION: MITOCHONDRIA Mitochondrial dynamics during spermatogenesis Grigor Varuzhanyan and David C. Chan* ABSTRACT Mitophagy is an additional layer of quality control that utilizes Mitochondrial fusion and fission (mitochondrial dynamics) are autophagy (Mizushima, 2007; Mizushima et al., 1998; Tsukada and homeostatic processes that safeguard normal cellular function. This Ohsumi, 1993) to remove excessive mitochondria or damaged relationship is especially strong in tissues with constitutively high energy mitochondria that are beyond repair (Pickles et al., 2018). During demands, such as brain, heart and skeletal muscle. Less is known about mitophagy, microtubule-associated protein 1A/1B light chain 3 the role of mitochondrial dynamics in developmental systems that involve (collectively LC3; also known as MAP1LC3A, MAP1LC3B and changes in metabolic function. One such system is spermatogenesis. MAP1LC3C) is recruited to the autophagosomal membrane and binds The first mitochondrial dynamics gene, Fuzzy onions (Fzo), was to mitochondria that selectively express mitophagy receptors on their discovered in 1997 to mediate mitochondrial fusion during Drosophila outer membrane. This molecular recognition designates damaged spermatogenesis. In mammals, however, the role of mitochondrial fusion mitochondria as cargo for autophagosomes, and the resulting during spermatogenesis remained unknown for nearly two decades after mitophagosomes subsequently fuse with lysosomes for degradation discovery of Fzo. Mammalian spermatogenesis is one of the most and recycling of the engulfed organelles. Much of the molecular complex and lengthy differentiation processes in biology, transforming workings of mitophagy have been parsed in cultured cells, and spermatogonial stem cells into highly specialized sperm cells over a recently developed mouse models are enabling the analysis of 5-week period. -

Comparative Analysis of a Teleost Skeleton Transcriptome Provides Insight Into Its Regulation
Accepted Manuscript Comparative analysis of a teleost skeleton transcriptome provides insight into its regulation Florbela A. Vieira, M.A.S. Thorne, K. Stueber, M. Darias, R. Reinhardt, M.S. Clark, E. Gisbert, D.M. Power PII: S0016-6480(13)00264-5 DOI: http://dx.doi.org/10.1016/j.ygcen.2013.05.025 Reference: YGCEN 11541 To appear in: General and Comparative Endocrinology Please cite this article as: Vieira, F.A., Thorne, M.A.S., Stueber, K., Darias, M., Reinhardt, R., Clark, M.S., Gisbert, E., Power, D.M., Comparative analysis of a teleost skeleton transcriptome provides insight into its regulation, General and Comparative Endocrinology (2013), doi: http://dx.doi.org/10.1016/j.ygcen.2013.05.025 This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain. 1 Comparative analysis of a teleost skeleton transcriptome 2 provides insight into its regulation 3 4 Florbela A. Vieira1§, M. A. S. Thorne2, K. Stueber3, M. Darias4,5, R. Reinhardt3, M. 5 S. Clark2, E. Gisbert4 and D. M. Power1 6 7 1Center of Marine Sciences, Universidade do Algarve, Faro, Portugal. 8 2British Antarctic Survey – Natural Environment Research Council, High Cross, 9 Madingley Road, Cambridge, CB3 0ET, UK. -

Supplementary Table 1: Adhesion Genes Data Set
Supplementary Table 1: Adhesion genes data set PROBE Entrez Gene ID Celera Gene ID Gene_Symbol Gene_Name 160832 1 hCG201364.3 A1BG alpha-1-B glycoprotein 223658 1 hCG201364.3 A1BG alpha-1-B glycoprotein 212988 102 hCG40040.3 ADAM10 ADAM metallopeptidase domain 10 133411 4185 hCG28232.2 ADAM11 ADAM metallopeptidase domain 11 110695 8038 hCG40937.4 ADAM12 ADAM metallopeptidase domain 12 (meltrin alpha) 195222 8038 hCG40937.4 ADAM12 ADAM metallopeptidase domain 12 (meltrin alpha) 165344 8751 hCG20021.3 ADAM15 ADAM metallopeptidase domain 15 (metargidin) 189065 6868 null ADAM17 ADAM metallopeptidase domain 17 (tumor necrosis factor, alpha, converting enzyme) 108119 8728 hCG15398.4 ADAM19 ADAM metallopeptidase domain 19 (meltrin beta) 117763 8748 hCG20675.3 ADAM20 ADAM metallopeptidase domain 20 126448 8747 hCG1785634.2 ADAM21 ADAM metallopeptidase domain 21 208981 8747 hCG1785634.2|hCG2042897 ADAM21 ADAM metallopeptidase domain 21 180903 53616 hCG17212.4 ADAM22 ADAM metallopeptidase domain 22 177272 8745 hCG1811623.1 ADAM23 ADAM metallopeptidase domain 23 102384 10863 hCG1818505.1 ADAM28 ADAM metallopeptidase domain 28 119968 11086 hCG1786734.2 ADAM29 ADAM metallopeptidase domain 29 205542 11085 hCG1997196.1 ADAM30 ADAM metallopeptidase domain 30 148417 80332 hCG39255.4 ADAM33 ADAM metallopeptidase domain 33 140492 8756 hCG1789002.2 ADAM7 ADAM metallopeptidase domain 7 122603 101 hCG1816947.1 ADAM8 ADAM metallopeptidase domain 8 183965 8754 hCG1996391 ADAM9 ADAM metallopeptidase domain 9 (meltrin gamma) 129974 27299 hCG15447.3 ADAMDEC1 ADAM-like, -

Predicting Gene Ontology Biological Process from Temporal Gene Expression Patterns Astrid Lægreid,1,4 Torgeir R
Methods Predicting Gene Ontology Biological Process From Temporal Gene Expression Patterns Astrid Lægreid,1,4 Torgeir R. Hvidsten,2 Herman Midelfart,2 Jan Komorowski,2,3,4 and Arne K. Sandvik1 1Department of Cancer Research and Molecular Medicine, Norwegian University of Science and Technology, N-7489 Trondheim, Norway; 2Department of Information and Computer Science, Norwegian University of Science and Technology, N-7491 Trondheim, Norway; 3The Linnaeus Centre for Bioinformatics, Uppsala University, SE-751 24 Uppsala, Sweden The aim of the present study was to generate hypotheses on the involvement of uncharacterized genes in biological processes. To this end,supervised learning was used to analyz e microarray-derived time-series gene expression data. Our method was objectively evaluated on known genes using cross-validation and provided high-precision Gene Ontology biological process classifications for 211 of the 213 uncharacterized genes in the data set used. In addition,new roles in biological process were hypothesi zed for known genes. Our method uses biological knowledge expressed by Gene Ontology and generates a rule model associating this knowledge with minimal characteristic features of temporal gene expression profiles. This model allows learning and classification of multiple biological process roles for each gene and can predict participation of genes in a biological process even though the genes of this class exhibit a wide variety of gene expression profiles including inverse coregulation. A considerable number of the hypothesized new roles for known genes were confirmed by literature search. In addition,many biological process roles hypothesi zed for uncharacterized genes were found to agree with assumptions based on homology information. -

Supplementary Table S4. FGA Co-Expressed Gene List in LUAD
Supplementary Table S4. FGA co-expressed gene list in LUAD tumors Symbol R Locus Description FGG 0.919 4q28 fibrinogen gamma chain FGL1 0.635 8p22 fibrinogen-like 1 SLC7A2 0.536 8p22 solute carrier family 7 (cationic amino acid transporter, y+ system), member 2 DUSP4 0.521 8p12-p11 dual specificity phosphatase 4 HAL 0.51 12q22-q24.1histidine ammonia-lyase PDE4D 0.499 5q12 phosphodiesterase 4D, cAMP-specific FURIN 0.497 15q26.1 furin (paired basic amino acid cleaving enzyme) CPS1 0.49 2q35 carbamoyl-phosphate synthase 1, mitochondrial TESC 0.478 12q24.22 tescalcin INHA 0.465 2q35 inhibin, alpha S100P 0.461 4p16 S100 calcium binding protein P VPS37A 0.447 8p22 vacuolar protein sorting 37 homolog A (S. cerevisiae) SLC16A14 0.447 2q36.3 solute carrier family 16, member 14 PPARGC1A 0.443 4p15.1 peroxisome proliferator-activated receptor gamma, coactivator 1 alpha SIK1 0.435 21q22.3 salt-inducible kinase 1 IRS2 0.434 13q34 insulin receptor substrate 2 RND1 0.433 12q12 Rho family GTPase 1 HGD 0.433 3q13.33 homogentisate 1,2-dioxygenase PTP4A1 0.432 6q12 protein tyrosine phosphatase type IVA, member 1 C8orf4 0.428 8p11.2 chromosome 8 open reading frame 4 DDC 0.427 7p12.2 dopa decarboxylase (aromatic L-amino acid decarboxylase) TACC2 0.427 10q26 transforming, acidic coiled-coil containing protein 2 MUC13 0.422 3q21.2 mucin 13, cell surface associated C5 0.412 9q33-q34 complement component 5 NR4A2 0.412 2q22-q23 nuclear receptor subfamily 4, group A, member 2 EYS 0.411 6q12 eyes shut homolog (Drosophila) GPX2 0.406 14q24.1 glutathione peroxidase -

3D Functional Corneal Stromal Tissue Equivalent Based on Corneal Stromal Stem Cells and Multi-Layered Silk Film Architecture
RESEARCH ARTICLE 3D Functional Corneal Stromal Tissue Equivalent Based on Corneal Stromal Stem Cells and Multi-Layered Silk Film Architecture Chiara E. Ghezzi1, Benedetto Marelli1, Fiorenzo G. Omenetto1, James L. Funderburgh2, David L. Kaplan1* 1 Department of Biomedical Engineering, Tufts University, Medford, Massachusetts, United States of America, 2 Department of Ophthalmology, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, United States of America * [email protected] a1111111111 a1111111111 a1111111111 Abstract a1111111111 a1111111111 The worldwide need for human cornea equivalents continues to grow. Few clinical options are limited to allogenic and synthetic material replacements. We hypothesized that tissue engineered human cornea systems based on mechanically robust, patterned, porous, thin, optically clear silk protein films, in combination with human corneal stromal stem cells OPEN ACCESS (hCSSCs), would generate 3D functional corneal stroma tissue equivalents, in comparison Citation: Ghezzi CE, Marelli B, Omenetto FG, to previously developed 2D approaches. Silk film contact guidance was used to control the Funderburgh JL, Kaplan DL (2017) 3D Functional alignment and distribution of hCSSCs on RGD-treated single porous silk films, which were Corneal Stromal Tissue Equivalent Based on then stacked in an orthogonally, multi-layered architecture and cultured for 9 weeks. These Corneal Stromal Stem Cells and Multi-Layered Silk Film Architecture. PLoS ONE 12(1): e0169504. systems were compared similar systems generated with human corneal fibroblasts (hCFs). doi:10.1371/journal.pone.0169504 Both cell types were viable and preferentially aligned along the biomaterial patterns for up to Editor: Dimitrios Karamichos, Oklahoma State 9 weeks in culture. H&E histological sections showed that the systems seeded with the University Center for Health Sciences, UNITED hCSSCs displayed ECM production throughout the entire thickness of the constructs. -
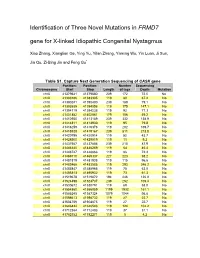
Identification of Three Novel Mutations in FRMD7 Gene for X-Linked Idiopathic Congenital Nystagmus
Identification of Three Novel Mutations in FRMD7 gene for X-linked Idiopathic Congenital Nystagmus Xiao Zhang, Xianglian Ge, Ying Yu, Yilan Zhang, Yaming Wu, Yin Luan, Ji Sun, Jia Qu, Zi-Bing Jin and Feng Gu* Table S1. Capture Next Generation Sequencing of CASK gene Position: Position: Number Sequencing Chromosome Start Stop Length of tags Depth Mutation chrX 41379641 41379880 239 172 72.0 No chrX 41383186 41383305 119 80 67.2 No chrX 41390241 41390480 239 189 79.1 No chrX 41393939 41394058 119 175 147.1 No chrX 41394119 41394238 119 92 77.3 No chrX 41401882 41402061 179 106 59.2 No chrX 41412950 41413189 239 332 138.9 No chrX 41414811 41414930 119 95 79.8 No chrX 41416259 41416378 119 202 169.7 No chrX 41418928 41419167 239 511 213.8 No chrX 41420795 41420914 119 52 43.7 No chrX 41428900 41429019 119 11 9.2 No chrX 41437567 41437806 239 210 87.9 No chrX 41446140 41446259 119 54 45.4 No chrX 41448747 41448866 119 86 72.3 No chrX 41469110 41469337 227 223 98.2 No chrX 41481819 41481938 119 115 96.6 No chrX 41483466 41483585 119 293 246.2 No chrX 41485847 41485966 119 75 63.0 No chrX 41495813 41495932 119 73 61.3 No chrX 41519678 41519872 194 246 126.8 No chrX 41524498 41524737 239 252 105.4 No chrX 41530672 41530791 119 69 58.0 No chrX 41554860 41556059 1199 1932 161.1 No chrX 41586245 41587324 1079 1044 96.8 No chrX 41598613 41598732 119 27 22.7 No chrX 41604756 41604875 119 27 22.7 No chrX 41646424 41646543 119 124 104.2 No chrX 41712364 41712483 119 37 31.1 No chrX 41782152 41782271 119 5 4.2 No Table S2. -

Application of Collagen I and IV in Bioengineering Transparent Ocular Tissues
REVIEW published: 26 August 2021 doi: 10.3389/fsurg.2021.639500 Application of Collagen I and IV in Bioengineering Transparent Ocular Tissues Yihui Song 1†, Morgan Overmass 1†, Jiawen Fan 2, Chris Hodge 1,3,4, Gerard Sutton 1,3,4, Frank J. Lovicu 1,5 and Jingjing You 1,6* 1 Save Sight Institute, Faculty of Medicine and Health, The University of Sydney, Sydney, NSW, Australia, 2 Key Laboratory of Myopia of State Health Ministry, Department of Ophthalmology and Vision Sciences, Eye and Ear, Nose, and Throat (ENT) Hospital, Shanghai Medical College, Fudan University, Shanghai, China, 3 New South Wales (NSW) Tissue Bank, Sydney, NSW, Australia, 4 Vision Eye Institute, Chatswood, NSW, Australia, 5 Discipline of Anatomy and Histology, School of Medical Sciences, The University of Sydney, Sydney, NSW, Australia, 6 School of Optometry and Vision Science, University of New South Wales, Sydney, NSW, Australia Collagens represent a major group of structural proteins expressed in different tissues and display distinct and variable properties. Whilst collagens are non-transparent in the skin, they confer transparency in the cornea and crystalline lens of the eye. There are 28 types of collagen that all share a common triple helix structure yet differ in the composition Edited by: of their α-chains leading to their different properties. The different organization of collagen Zhilian Yue, fibers also contributes to the variable tissue morphology. The important ability of collagen University of Wollongong, Australia to form different tissues has led to the exploration and application of collagen as a Reviewed by: biomaterial. Collagen type I (Col-I) and collagen type IV (Col-IV) are the two primary Tiago H. -

Nº Ref Uniprot Proteína Péptidos Identificados Por MS/MS 1 P01024
Document downloaded from http://www.elsevier.es, day 26/09/2021. This copy is for personal use. Any transmission of this document by any media or format is strictly prohibited. Nº Ref Uniprot Proteína Péptidos identificados 1 P01024 CO3_HUMAN Complement C3 OS=Homo sapiens GN=C3 PE=1 SV=2 por 162MS/MS 2 P02751 FINC_HUMAN Fibronectin OS=Homo sapiens GN=FN1 PE=1 SV=4 131 3 P01023 A2MG_HUMAN Alpha-2-macroglobulin OS=Homo sapiens GN=A2M PE=1 SV=3 128 4 P0C0L4 CO4A_HUMAN Complement C4-A OS=Homo sapiens GN=C4A PE=1 SV=1 95 5 P04275 VWF_HUMAN von Willebrand factor OS=Homo sapiens GN=VWF PE=1 SV=4 81 6 P02675 FIBB_HUMAN Fibrinogen beta chain OS=Homo sapiens GN=FGB PE=1 SV=2 78 7 P01031 CO5_HUMAN Complement C5 OS=Homo sapiens GN=C5 PE=1 SV=4 66 8 P02768 ALBU_HUMAN Serum albumin OS=Homo sapiens GN=ALB PE=1 SV=2 66 9 P00450 CERU_HUMAN Ceruloplasmin OS=Homo sapiens GN=CP PE=1 SV=1 64 10 P02671 FIBA_HUMAN Fibrinogen alpha chain OS=Homo sapiens GN=FGA PE=1 SV=2 58 11 P08603 CFAH_HUMAN Complement factor H OS=Homo sapiens GN=CFH PE=1 SV=4 56 12 P02787 TRFE_HUMAN Serotransferrin OS=Homo sapiens GN=TF PE=1 SV=3 54 13 P00747 PLMN_HUMAN Plasminogen OS=Homo sapiens GN=PLG PE=1 SV=2 48 14 P02679 FIBG_HUMAN Fibrinogen gamma chain OS=Homo sapiens GN=FGG PE=1 SV=3 47 15 P01871 IGHM_HUMAN Ig mu chain C region OS=Homo sapiens GN=IGHM PE=1 SV=3 41 16 P04003 C4BPA_HUMAN C4b-binding protein alpha chain OS=Homo sapiens GN=C4BPA PE=1 SV=2 37 17 Q9Y6R7 FCGBP_HUMAN IgGFc-binding protein OS=Homo sapiens GN=FCGBP PE=1 SV=3 30 18 O43866 CD5L_HUMAN CD5 antigen-like OS=Homo -

Safety and Efficacy of Combination of Suberoylamilide Hydroxyamic Acid
www.nature.com/scientificreports OPEN Safety and efcacy of combination of suberoylamilide hydroxyamic acid and mitomycin C in reducing pro‑fbrotic changes in human corneal epithelial cells Rohit Shetty1,7, Nimisha Rajiv Kumar2,7, Murali Subramani3, Lekshmi Krishna3, Ponnalagu Murugeswari3, Himanshu Matalia1, Pooja Khamar1, Zelda V. Dadachanji1, Rajiv R. Mohan4,5,6*, Arkasubhra Ghosh2* & Debashish Das3* Corneal haze post refractive surgery is prevented by mitomycin c (MMC) treatment though it can lead to corneal endothelial damage, persistent epithelial defects and necrosis of cells. Suberanilohydroxamic acid (SAHA) however has been proposed to prevent corneal haze without any adverse efects. For clinical application we have investigated the short and long term outcome of cells exposed to SAHA. Human donor cornea, cultured limbal epithelial cells, corneal rims and lenticules were incubated with SAHA and MMC. The cells/tissue was then analyzed by RT‑qPCR, immunofuorescence and western blot for markers of apoptosis and fbrosis. The results reveal that short term exposure of SAHA and SAHA + MMC reduced apoptosis levels and increased αSMA expression compared to those treated with MMC. Epithelial cells derived from cultured corneal rim that were incubated with the MMC, SAHA or MMC + SAHA revealed enhanced apoptosis, reduced levels of CK3/CK12, ∆NP63 and COL4A compared to other treatments. In SAHA treated lenticules TGFβ induced fbrosis was reduced. The results imply that MMC treatment for corneal haze has both short term and long term adverse efects on cells and the cellular properties. However, a combinatorial treatment of SAHA + MMC prevents expression of corneal fbrotic markers without causing any adverse efect on cellular properties. -

Regulation of COX Assembly and Function by Twin CX9C Proteins—Implications for Human Disease
cells Review Regulation of COX Assembly and Function by Twin CX9C Proteins—Implications for Human Disease Stephanie Gladyck 1, Siddhesh Aras 1,2, Maik Hüttemann 1 and Lawrence I. Grossman 1,2,* 1 Center for Molecular Medicine and Genetics, Wayne State University School of Medicine, Detroit, MI 48201, USA; [email protected] (S.G.); [email protected] (S.A.); [email protected] (M.H.) 2 Perinatology Research Branch, Division of Obstetrics and Maternal-Fetal Medicine, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U.S. Department of Health and Human Services, Bethesda, Maryland and Detroit, MI 48201, USA * Correspondence: [email protected] Abstract: Oxidative phosphorylation is a tightly regulated process in mammals that takes place in and across the inner mitochondrial membrane and consists of the electron transport chain and ATP synthase. Complex IV, or cytochrome c oxidase (COX), is the terminal enzyme of the electron transport chain, responsible for accepting electrons from cytochrome c, pumping protons to contribute to the gradient utilized by ATP synthase to produce ATP, and reducing oxygen to water. As such, COX is tightly regulated through numerous mechanisms including protein–protein interactions. The twin CX9C family of proteins has recently been shown to be involved in COX regulation by assisting with complex assembly, biogenesis, and activity. The twin CX9C motif allows for the import of these proteins into the intermembrane space of the mitochondria using the redox import machinery of Mia40/CHCHD4. Studies have shown that knockdown of the proteins discussed in this review results in decreased or completely deficient aerobic respiration in experimental models ranging from yeast to human cells, as the proteins are conserved across species.