Insights from the Malagasy Canellales
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Outline of Angiosperm Phylogeny
Outline of angiosperm phylogeny: orders, families, and representative genera with emphasis on Oregon native plants Priscilla Spears December 2013 The following listing gives an introduction to the phylogenetic classification of the flowering plants that has emerged in recent decades, and which is based on nucleic acid sequences as well as morphological and developmental data. This listing emphasizes temperate families of the Northern Hemisphere and is meant as an overview with examples of Oregon native plants. It includes many exotic genera that are grown in Oregon as ornamentals plus other plants of interest worldwide. The genera that are Oregon natives are printed in a blue font. Genera that are exotics are shown in black, however genera in blue may also contain non-native species. Names separated by a slash are alternatives or else the nomenclature is in flux. When several genera have the same common name, the names are separated by commas. The order of the family names is from the linear listing of families in the APG III report. For further information, see the references on the last page. Basal Angiosperms (ANITA grade) Amborellales Amborellaceae, sole family, the earliest branch of flowering plants, a shrub native to New Caledonia – Amborella Nymphaeales Hydatellaceae – aquatics from Australasia, previously classified as a grass Cabombaceae (water shield – Brasenia, fanwort – Cabomba) Nymphaeaceae (water lilies – Nymphaea; pond lilies – Nuphar) Austrobaileyales Schisandraceae (wild sarsaparilla, star vine – Schisandra; Japanese -

Lessons from 20 Years of Plant Genome Sequencing: an Unprecedented Resource in Need of More Diverse Representation
bioRxiv preprint doi: https://doi.org/10.1101/2021.05.31.446451; this version posted May 31, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Lessons from 20 years of plant genome sequencing: an unprecedented resource in need of more diverse representation Authors: Rose A. Marks1,2,3, Scott Hotaling4, Paul B. Frandsen5,6, and Robert VanBuren1,2 1. Department of Horticulture, Michigan State University, East Lansing, MI 48824, USA 2. Plant Resilience Institute, Michigan State University, East Lansing, MI 48824, USA 3. Department of Molecular and Cell Biology, University of Cape Town, Rondebosch 7701, South Africa 4. School of Biological Sciences, Washington State University, Pullman, WA, USA 5. Department of Plant and Wildlife Sciences, Brigham Young University, Provo, UT, USA 6. Data Science Lab, Smithsonian Institution, Washington, DC, USA Keywords: plants, embryophytes, genomics, colonialism, broadening participation Correspondence: Rose A. Marks, Department of Horticulture, Michigan State University, East Lansing, MI 48824, USA; Email: [email protected]; Phone: (603) 852-3190; ORCID iD: https://orcid.org/0000-0001-7102-5959 Abstract The field of plant genomics has grown rapidly in the past 20 years, leading to dramatic increases in both the quantity and quality of publicly available genomic resources. With an ever- expanding wealth of genomic data from an increasingly diverse set of taxa, unprecedented potential exists to better understand the evolution and genome biology of plants. -

Phylogenomic Approach
Toward the ultimate phylogeny of Magnoliaceae: phylogenomic approach Sangtae Kim*1, Suhyeon Park1, and Jongsun Park2 1 Sungshin University, Korea 2 InfoBoss Co., Korea Mr. Carl Ferris Miller Founder of Chollipo Arboretum in Korea Chollipo Arboretum Famous for its magnolia collection 2020. Annual Meeting of Magnolia Society International Cholliop Arboretum in Korea. April 13th~22th, 2020 http://WWW.Chollipo.org Sungshin University, Seoul, Korea Dr. Hans Nooteboom Dr. Liu Yu-Hu Twenty-one years ago... in 1998 The 1st International Symposium on the Family Magnoliaceae, Gwangzhow Dr. Hiroshi Azuma Mr. Richard Figlar Dr. Hans Nooteboom Dr. Qing-wen Zeng Dr. Weibang Sun Handsome young boy Dr. Yong-kang Sima Dr. Yu-wu Law Presented ITS study on Magnoliaceae - never published Ten years ago... in 2009 Presented nine cp genome region study (9.2 kbp) on Magnoliaceae – published in 2013 2015 1st International Sympodium on Neotropical Magnoliaceae Gadalajara, 2019 3rd International Sympodium and Workshop on Neotropical Magnoliaceae Asterales Dipsacales Apiales Why magnolia study is Aquifoliales Campanulids (Euasterids II) Garryales Gentianales Laminales Solanales Lamiids important in botany? Ericales Asterids (Euasterids I) Cornales Sapindales Malvales Brassicales Malvids Fagales (Eurosids II) • As a member of early-diverging Cucurbitales Rosales Fabales Zygophyllales Celestrales Fabids (Eurosid I) angiosperms, reconstruction of the Oxalidales Malpighiales Vitales Geraniales Myrtales Rosids phylogeny of Magnoliaceae will Saxifragales Caryphyllales -

And According to Bailey & (1943)
BLUMEA 24 (1978) 521—525 The Winteraceae pf the Old World. III. Notes on the ovary of Takhtajania W. Vink Rijksherbarium, Leiden, Netherlands INTRODUCTION Very recently Baranova & Leroy (Leroy, 1978) published a new genus Takhta- jania to accomodate the aberrant Bubbia perrieri Capuron. The outstanding characters of this genus are the anomocytic stomatal apparatus (Baranova, 1972; Bongers, 1973) and the unilocular bicarpellate ovary (Leroy, 1977, 1978). work the Winteraceae, also studied the of Bub- During my on I single specimen bia perrieri in existence. The late Capuron told me that he had tried to collect additional specimens but that he had not succeeded in doing so; according to him the type locality was completely deforested. In view of the scarcity of the material it was considered relevant to publish some additional notes without delay. MATERIAL AND METHODS I studied three ovaries of the type specimen (Perrier de la Bdthie 10158). One was cut longitudinally, slightly outside the plane of symmetry; another was cut and its half These drawn transversally upper again longitudinally. parts were (figs. 2 and 3) and then cleared according to Bailey & Nast (1943) (figs 5 and third boiled and then sectioned 6). The ovary was serially (fig. 4). OBSERVATIONS One of the ovaries was taken from a flowerbud of which the closed petals were 57 mm high. The diagram of this flowerbud is drawn in fig. 1. The 2 slightly ruptured calyx showed three putative original apices ± alternating with the three bracteoles at the base of the pedicel. The petals are all free. The ovary is its and the flattened, has a longitudinal groove on narrow sides, highest sta- mens are inserted adjoining its broadest sides. -

Reconstructing the Basal Angiosperm Phylogeny: Evaluating Information Content of Mitochondrial Genes
55 (4) • November 2006: 837–856 Qiu & al. • Basal angiosperm phylogeny Reconstructing the basal angiosperm phylogeny: evaluating information content of mitochondrial genes Yin-Long Qiu1, Libo Li, Tory A. Hendry, Ruiqi Li, David W. Taylor, Michael J. Issa, Alexander J. Ronen, Mona L. Vekaria & Adam M. White 1Department of Ecology & Evolutionary Biology, The University Herbarium, University of Michigan, Ann Arbor, Michigan 48109-1048, U.S.A. [email protected] (author for correspondence). Three mitochondrial (atp1, matR, nad5), four chloroplast (atpB, matK, rbcL, rpoC2), and one nuclear (18S) genes from 162 seed plants, representing all major lineages of gymnosperms and angiosperms, were analyzed together in a supermatrix or in various partitions using likelihood and parsimony methods. The results show that Amborella + Nymphaeales together constitute the first diverging lineage of angiosperms, and that the topology of Amborella alone being sister to all other angiosperms likely represents a local long branch attrac- tion artifact. The monophyly of magnoliids, as well as sister relationships between Magnoliales and Laurales, and between Canellales and Piperales, are all strongly supported. The sister relationship to eudicots of Ceratophyllum is not strongly supported by this study; instead a placement of the genus with Chloranthaceae receives moderate support in the mitochondrial gene analyses. Relationships among magnoliids, monocots, and eudicots remain unresolved. Direct comparisons of analytic results from several data partitions with or without RNA editing sites show that in multigene analyses, RNA editing has no effect on well supported rela- tionships, but minor effect on weakly supported ones. Finally, comparisons of results from separate analyses of mitochondrial and chloroplast genes demonstrate that mitochondrial genes, with overall slower rates of sub- stitution than chloroplast genes, are informative phylogenetic markers, and are particularly suitable for resolv- ing deep relationships. -

Early Vessel Evolution and the Diverisification
American Journal of Botany 97(1): 80–93. 2010. E ARLY VESSEL EVOLUTION AND THE DIVERISIFICATION OF WOOD FUNCTION: INSIGHTS FROM MALAGASY CANELLALES 1 Patrick J. Hudson 2 , Jacqueline Razanatsoa 3 , and Taylor S. Feild 2,4 2 Department of Ecology and Evolutionary Biology, University of Tennessee, Knoxville, Tennessee 37919 USA; and 3 Herbier du Parc Botanique et Zoologique de Tsimbazaza, Rue Fernand, Kasanga, Tsimbazaza, Antananarivo 101, Madagascar Xylem vessels have long been proposed as a key innovation for the ecological diversifi cation of angiosperms by providing a breakthrough in hydraulic effi ciency to support high rates of photosynthesis and growth. However, recent studies demonstrated that angiosperm woods with structurally “ primitive ” vessels did not have greater whole stem hydraulic capacities as compared to vesselless angiosperms. As an alternative to the hydraulic superiority hypothesis, the heteroxylly hypothesis proposes that subtle hydraulic effi ciencies of primitive vessels over tracheids enabled new directions of functional specialization in the wood. However, the functional properties of early heteroxyllous wood remain unknown. We selected the two species of Canellales from Madagascar to test the heteroxylly hypothesis because Canellaceae (represented by Cinnamosma madagascariensis ) produces wood with vessels of an ancestral form, while Winteraceae, the sister clade (represented by Takhtajania perrieri) is vesselless. We found that heteroxy- lly correlated with increased wood functional diversity related predominantly to biomechanical specialization. However, vessels were not associated with greater stem hydraulic effi ciency or increased shoot hydraulic capacity. Our results support the heteroxylly hypothesis and highlight the importance integrating a broader ecological context to understand the evolution of vessels. Key words: Canellales; heteroxylly; hydraulic conductivity; shade tolerance; xylem evolution; vessel development. -

Guide to Theecological Systemsof Puerto Rico
United States Department of Agriculture Guide to the Forest Service Ecological Systems International Institute of Tropical Forestry of Puerto Rico General Technical Report IITF-GTR-35 June 2009 Gary L. Miller and Ariel E. Lugo The Forest Service of the U.S. Department of Agriculture is dedicated to the principle of multiple use management of the Nation’s forest resources for sustained yields of wood, water, forage, wildlife, and recreation. Through forestry research, cooperation with the States and private forest owners, and management of the National Forests and national grasslands, it strives—as directed by Congress—to provide increasingly greater service to a growing Nation. The U.S. Department of Agriculture (USDA) prohibits discrimination in all its programs and activities on the basis of race, color, national origin, age, disability, and where applicable sex, marital status, familial status, parental status, religion, sexual orientation genetic information, political beliefs, reprisal, or because all or part of an individual’s income is derived from any public assistance program. (Not all prohibited bases apply to all programs.) Persons with disabilities who require alternative means for communication of program information (Braille, large print, audiotape, etc.) should contact USDA’s TARGET Center at (202) 720-2600 (voice and TDD).To file a complaint of discrimination, write USDA, Director, Office of Civil Rights, 1400 Independence Avenue, S.W. Washington, DC 20250-9410 or call (800) 795-3272 (voice) or (202) 720-6382 (TDD). USDA is an equal opportunity provider and employer. Authors Gary L. Miller is a professor, University of North Carolina, Environmental Studies, One University Heights, Asheville, NC 28804-3299. -

2003-2004 Recovery Report to Congress
U.S. Fish & Wildlife Service Report to Congress on the Recovery of Threatened and Endangered Species Fiscal Years 2003-2004 U.S. Fish & Wildlife Service Endangered Species Program www.fws.gov/endangered December 2006 The U.S. Fish and Wildlife Service is responsible under the Endangered Species Act for conserving and recovering our nation’s rarest plant and animal species and their habitats, working in cooperation with other public and private partners. From the Director Endangered Species Program Contacts Do you want more information on a particular threatened or endangered species or recovery effort near you? Please contact the Regional Office that covers the This 2004 report provides an update on the State(s) you are interested in. If they cannot help you, they will gladly direct you recovery of threatened and endangered species to the nearest Service office. for the period between October 1, 2002, and Region Six — Mountain-Prairie September 30, 2004, and chronicles the progress Washington D.C. Office Region Four — Southeast 134 Union Boulevard, Suite 650 of efforts by the Fish and Wildlife Service and Endangered Species Program 1875 Century Boulevard, Suite 200 Lakewood, CO 80228 the many partners involved in recovery efforts. 4401 N. Fairfax Drive, Room 420 Atlanta, GA 30345 http://mountain-prairie.fws.gov/endspp Arlington, VA 22203 http://www.fws.gov/southeast/es/ During this time, recovery efforts enabled three http://www.fws.gov/endangered Chief, Division of Ecological Services: species to be removed from the Endangered and Chief, -
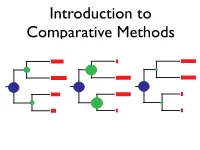
BM CC EB What Can We Learn from a Tree?
Introduction to Comparative Methods BM CC EB What can we learn from a tree? Net diversification (r) Relative extinction (ε) Peridiscaceae Peridiscaceae yllaceae yllaceae h h atop atop Proteaceae Proteaceae r r Ce Ce Tr oc T ho r M M o de c y y H H C C h r r e e a D D a o o nd o e e G G a a m m t t a a d r P r P h h e e u u c c p A p A r e a a e e a a a c a c n n a i B i B h h n d m d l m a l a m m e a e a e e t t n n c u u n n i i d i i e e e e o n o n p n p n a e a S e e S e e n n x x i i r c c a a n o n o p p h g e h g ae e l r a l r a a a a a a i i a a a e a e i b i b h y d c h d c i y i c a a c x c x c c G I a G I a n c n c c c y l y l t a a t a a e e e e e i l c i l c m l m l e c e c f a e a a f a e a a l r r l c c a i i r l e t e t a a r l a a e e u u u u o a o a a a c a c a a l a l e e e b b a a a a e e c e e c a a s c s c c e l c e l e e g e g e a a a a e e n n s e e s e e e e a a a P a P e e N N u u u S u S a e a e a a e e c c l n a l n e e a e e a e a e a e a e r a r a c c C i C i R R a e a e a e a e r c r c A A a d a a d a e i e i phanopetalaceae s r e ph s r e a a s e c s e c e e u u b a a b a e e P P r r l l e n e a a a a m m entho e e e Ha a H o a c r e c r e nt B B e p e e e c e e c a c c h e a p a a p a lo lo l l a a e s o t e s i a r a i a r r r r r a n e a n e a b a l b t a t gaceae e g e ceae a c a s c s a z e z M i a e M i a c a d e a d e ae e ae r e r a e a e a a a c c ce r e r L L i i ac a Vitaceae Vi r r C C e e ta v e v e a a c a a e ea p e ap c a c a e a P P e e l l e Ge G e e ae a t t e e p p r r ce c an u an -

Supplementary Information
Supporting Information for Cornwell et al. 2014. Functional distinctiveness of major 1 plant lineages. doi: 10.1111/1365-2745.12208. SUPPORTING INFORMATION Summary In this Supporting Information, we report additional methods details with respect to the trait data, the climate data, and the analysis. In addition, to more fully explain the method, we include several additional figures. We show the top-five lineages, including the population of lineages from which they were selected, for five important traits (Figure S1 on five separate pages); the bivariate distribution of three selected clades with respect to SLA and leaf N, components of the leaf economic spectrum (Figure S2), the geographic distribution of the clades where this proved useful for interpretation (Figure S3); the procedure for selecting the top six nodes in the leaf N trait, to illustrate the internal behavior of our new comparative method, especially the relative contribution of components of extremeness and the sample size weighting to the results (Figure S4). Finally, we provide references for data used in analyses. SUPPORTING METHODS TRAITS DATABASE We compiled a database for five plant functional traits. Each of these traits is the result of a separate research initiative in which data were gathered directly from researchers leading those efforts and/or the literature; in most, but not all cases, these data have been published elsewhere. Detailed methods for data collection and assembly for each trait are available in the original publications; further data were added for some traits from the primary literature (for references see description of individual traits and Supporting References below). For our compilation, all data were brought to common units for a given trait and thoroughly error checked. -

Insecticidal and Antifeedant Activities of Malagasy Medicinal Plant (Cinnamosma Sp.) Extracts and Drimane-Type Sesquiterpenes Against Aedes Aegypti Mosquitoes
Insecticidal and antifeedant activities of Malagasy medicinal plant (Cinnamosma sp.) extracts and drimane-type sesquiterpenes against Aedes aegypti mosquitoes Thesis Presented in Partial Fulfillment of the Requirements for the Degree Master of Science in the Graduate School of The Ohio State University By Edna Ariel Alfaro Inocente, B.S. Graduate Program in Entomology The Ohio State University 2020 Thesis Committee Dr. Peter M. Piermarini, Advisor Dr. Reed M. Johnson Dr. Liva H. Rakotondraibe Copyrighted by Edna Ariel Alfaro Inocente 2020 2 Abstract Nearly everyone has been bitten at least once by a mosquito and recognizes how annoying this can be. But mosquito bites are nothing compared to the number of people that have died from and continue to be at risk of contracting arboviruses transmitted by these deadly insects. Female Aedes aegypti mosquitoes are the primary vector of the viruses that cause yellow fever, dengue fever, chikungunya fever, and Zika virus in humans. Climate change and globalization have facilitated the spread of these mosquitoes and associated arboviruses, generating new outbreaks and increasing disease risk in the United States. Effective vaccines or medical treatments for most mosquito-borne arboviruses are not available and thereby the most common strategy to control viral transmission is to manage mosquito populations with chemical pesticides and prevent mosquito-human interactions with chemical repellents. Although the use of neurotoxic chemicals, such as organophosphates and pyrethroids, can be very efficient at killing mosquitoes, the limited modes of action of these compounds has strongly selected for individuals that are resistant. Likewise, the chemical arsenal for mosquito repellents is limited. -

Intercontinental Long-Distance Dispersal of Canellaceae from the New to the Old World Revealed by a Nuclear Single Copy Gene and Chloroplast Loci
Molecular Phylogenetics and Evolution 84 (2015) 205–219 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev Intercontinental long-distance dispersal of Canellaceae from the New to the Old World revealed by a nuclear single copy gene and chloroplast loci Sebastian Müller a,1, Karsten Salomo a,1, Jackeline Salazar b, Julia Naumann a, M. Alejandra Jaramillo c, ⇑ Christoph Neinhuis a, Taylor S. Feild d,2, Stefan Wanke a, ,2 a Technische Universität Dresden, Institut für Botanik, Zellescher Weg 20b, 01062 Dresden, Germany b Escuela de Biología, Universidad Autónoma de Santo Domingo (UASD), C/Bartolomé Mitre, Santo Domingo, Dominican Republic c Centro de Investigación para el Manejo Ambiental y el Desarrollo, Cali, Colombia d Centre for Tropical Biodiversity and Climate Change, College of Marine and Environmental Science, Townsville 4810, Campus Townsville, Australia article info abstract Article history: Canellales, a clade consisting of Winteraceae and Canellaceae, represent the smallest order of magnoliid Received 10 July 2014 angiosperms. The clade shows a broad distribution throughout the Southern Hemisphere, across a diverse Revised 16 December 2014 range of dry to wet tropical forests. In contrast to their sister-group, Winteraceae, the phylogenetic rela- Accepted 17 December 2014 tions and biogeography within Canellaceae remain poorly studied. Here we present the phylogenetic Available online 9 January 2015 relationships of all currently recognized genera of Canellales with a special focus on the Old World Canellaceae using a combined dataset consisting of the chloroplast trnK-matK-trnK-psbA and the nuclear Keywords: single copy gene mag1 (Maigo 1). Within Canellaceae we found high statistical support for the mono- Canellales phyly of Warburgia and Cinnamosma.