Ecological Impacts of Invasive Carp in Australian Dryland Rivers
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fish Assemblages of an Australian Dryland River: Abundance, Assemblage Structure and Recruitment Patterns in the Warrego River, Murray–Darling Basin
CSIRO PUBLISHING www.publish.csiro.au/journals/mfr Marine and Freshwater Research, 2006, 57, 619–633 Fish assemblages of an Australian dryland river: abundance, assemblage structure and recruitment patterns in the Warrego River, Murray–Darling Basin Stephen R. BalcombeA,E, Angela H. ArthingtonA, Neal D. FosterB, Martin C. ThomsC, Glenn G. WilsonD and Stuart E. BunnA ACooperative Research Centre For Freshwater Ecology, Centre for Riverine Landscapes, Griffith University, Nathan, Qld 4111, Australia. BNew South Wales Department of Natural Resources, PO Box 550, Tamworth, NSW 2340, Australia. CUniversity of Canberra, ACT 2016, Australia. DMurray–Darling Freshwater Research Centre, Goondiwindi, Qld 4390, Australia. ECorresponding author. Email: [email protected] Abstract. Fish in dryland rivers must cope with extreme variability in hydrology, temperature and other environ- mental factors that ultimately have a major influence on their patterns of distribution and abundance at the landscape scale. Given that fish persist in these systems under conditions of high environmental variability, dryland rivers represent ideal systems to investigate the processes contributing to and sustaining fish biodiversity and recruitment in variable environments. Hence, spatial and temporal variation in fish assemblage structure was examined in 15 waterholes of the Warrego River between October 2001 and May 2003. Fish assemblages in isolated waterholes were differentiated at the end of the dry 2001 winter but were relatively similar following high summer flows in January 2002 as a consequence of high hydrological connectivity among waterholes. Small, shallow waterholes supported more species and higher abundances than large-deep waterholes. Large, deep waterholes provided impor- tant refuge for large-bodied fish species such as adult yellowbelly, Macquaria ambigua, and the eel-tailed catfish, Tandanus tandanus. -

Warrego River Scoping Study – Summary
FinalWestern Summary Catchment Management Authority WarregoFinal River Summary Scoping Study 2008 WESTERN Contact Details Western CMA Offices: 45 Wingewarra Street Dubbo NSW 2830 Ph 02 6883 3000 32 Sulphide Street Broken Hill NSW 2880 Ph 08 8082 5200 21 Mitchell Street Bourke NSW 2840 Ph 02 6872 2144 62 Marshall Street Cobar NSW 2835 Ph 02 6836 1575 89 Wee Waa Street Walgett NSW 2832 Ph 02 6828 0110 Freecall 1800 032 101 www.western.cma.nsw.gov.au WMA Head Office: Level 2, 160 Clarence Street Sydney NSW 2000 Ph 02 9299 2855 Fax 02 9262 6208 E [email protected] W www.wmawater.com.au warrego river scoping study - page 2 1. Warrego River Scoping Study – Summary 1.1. Project Rationale and Objectives The Queensland portion of the Warrego is managed under the Warrego, Paroo, Bulloo and Nebine Water Resource Plan (WRP) (2003) and the Resource Operation Plan (ROP) (2006). At present, there is no planning instrument in NSW. As such, there was concern in the community that available information be consolidated and assessed for usability for the purposes of supporting development of a plan in NSW and for future reviews of the QLD plan. There was also concern that an assessment be undertaken of current hydrologic impacts due to water resource development and potential future impacts. As a result, the Western Catchment Management Authority (WCMA) commissioned WMAwater to undertake a scoping study for the Warrego River and its tributaries and effluents. This study was to synthesise and identify gaps in knowledge / data relating to hydrology, flow dependent environmental assets and water planning instruments. -

Southern Gulf, Queensland
Biodiversity Summary for NRM Regions Species List What is the summary for and where does it come from? This list has been produced by the Department of Sustainability, Environment, Water, Population and Communities (SEWPC) for the Natural Resource Management Spatial Information System. The list was produced using the AustralianAustralian Natural Natural Heritage Heritage Assessment Assessment Tool Tool (ANHAT), which analyses data from a range of plant and animal surveys and collections from across Australia to automatically generate a report for each NRM region. Data sources (Appendix 2) include national and state herbaria, museums, state governments, CSIRO, Birds Australia and a range of surveys conducted by or for DEWHA. For each family of plant and animal covered by ANHAT (Appendix 1), this document gives the number of species in the country and how many of them are found in the region. It also identifies species listed as Vulnerable, Critically Endangered, Endangered or Conservation Dependent under the EPBC Act. A biodiversity summary for this region is also available. For more information please see: www.environment.gov.au/heritage/anhat/index.html Limitations • ANHAT currently contains information on the distribution of over 30,000 Australian taxa. This includes all mammals, birds, reptiles, frogs and fish, 137 families of vascular plants (over 15,000 species) and a range of invertebrate groups. Groups notnot yet yet covered covered in inANHAT ANHAT are notnot included included in in the the list. list. • The data used come from authoritative sources, but they are not perfect. All species names have been confirmed as valid species names, but it is not possible to confirm all species locations. -

Diversity Patterns in Australia
Provided for non-commercial research and educational use only. Not for reproduction, distribution or commercial use. This chapter was originally published in the book Encyclopedia of Caves, published by Elsevier, and the attached copy is provided by Elsevier for the author’s benefit and for the benefit of the author’s institution, for non-commercial research and educational use including without limitation use in instruction at your institution, sending it to specific colleagues who know you, and providing a copy to your institution’s administrator. All other uses, reproduction and distribution, including without limitation commercial reprints, selling or licensing copies or access, or posting on open internet sites, your personal or institution’s website or repository, are prohibited. For exceptions, permission may be sought for such use through Elsevier’s permissions site at: http://www.elsevier.com/locate/permissionusematerial From William F. Humphreys, Diversity Patterns in Australia. In: William B. White and David C. Culver, editors, Encyclopedia of Caves. Chennai: Academic Press, 2012, pp. 203-219. ISBN: 978-0-12-383832-2 Copyright © 2012 Elsevier Inc. Academic Press. Author’s personal copy DIVERSITY PATTERNS IN AUSTRALIA 203 Gams, I., & Gabrovec, M. (1999). Land use and human impact in the Dinaric karst. International Journal of Speleology, 28B(1À4), 55À77. Habic,ˇ P. (1991). Geomorphological classification of NW Dinaric karst. Acta Carsologica, 20, 133À164. Kranjc, A. (2008). History of deforestation and reforestation in the Dinaric karst. Geographical Research, 47(1), 15À23. Mihevc, A. (2007). The age of karst relief in West Slovenia. Acta Carsologica, 36(1), 35À44. Milanovic,´ P. T. (1981). Karst hydrology. -

South West Queensland Floods March 2010
South West Queensland Floods March 2010 1 2 3 4 5 6 7 8 1. Floodwaters inundate the township of Bollon. Photo supplied by Bill Speedy. 2. Floodwaters at the Autumnvale gauging station on the lower Bulloo River. Photo supplied by R.D. & C.B. Hughes. 3. Floodwaters from Bradley’s Gully travel through Charleville. 4. Floodwaters from Bungil Creek inundate Roma. Photo supplied by the Maranoa Regional Council. 5. Floodwaters at the confluence of the Paroo River and Beechal Creek. Photo supplied by Cherry and John Gardiner. 6. Balonne River floodwaters inundate low lying areas of St. George. Photo supplied by Sally Nichol. 7. Floodwaters from the Moonie River inundate Nindigully. Photo supplied by Sally Nichol. 8. Floodwaters from the Moonie River inundate the township of Thallon. Photo supplied by Sally Nichol. Revision history Date Version Description 6 June 2010 1.0 Original Original version of this report contained an incorrect date for the main flood peak at Roma. Corrected to 23 June 2010 1.1 8.1 metres on Tuesday 2 March 2010. See Table 3.1.1. An approximate peak height has been replaced for Bradley’s Gully at Charleville. New peak height is 4.2 28 June 2010 1.2 metres on Tuesday 2 March 2010 at 13:00. See Table 3.1.1. Peak height provided from flood mark at Teelba on 01 July 2010 1.3 Teelba Creek. See Table 3.1.1. 08 Spectember Peak height provided from flood mark at Garrabarra 1.4 2010 on Bungil Creek. See Table 3.1.1. -

Resistance and Resilience of Murray-Darling Basin Fishes to Drought Disturbance
Resistance and Resilience of Murray- Darling Basin Fishes to Drought Disturbance Dale McNeil1, Susan Gehrig1 and Clayton Sharpe2 SARDI Publication No. F2009/000406-1 SARDI Research Report Series No. 602 SARDI Aquatic Sciences PO Box 120 Henley Beach SA 5022 April 2013 Final Report to the Murray-Darling Basin Authority - Native Fish Strategy Project MD/1086 “Ecosystem Resilience and the Role of Refugia for Native Fish Communities & Populations” McNeil et. al. 2013 Drought and Native Fish Resilience Resistance and Resilience of Murray- Darling Basin Fishes to Drought Disturbance Final Report to the Murray-Darling Basin Authority - Native Fish Strategy Project MD/1086 “Ecosystem Resilience and the Role of Refugia for Native Fish Communities & Populations” Dale McNeil1, Susan Gehrig1 and Clayton Sharpe2 SARDI Publication No. F2009/000406-1 SARDI Research Report Series No. 602 April 2013 Page | ii McNeil et. al. 2013 Drought and Native Fish Resilience This Publication may be cited as: McNeil, D. G., Gehrig, S. L. and Sharpe, C. P. (2013). Resistance and Resilience of Murray-Darling Basin Fishes to Drought Disturbance. Final Report to the Murray-Darling Basin Authority - Native Fish Strategy Project MD/1086 ―Ecosystem Resilience and the Role of Refugia for Native Fish Communities & Populations‖. South Australian Research and Development Institute (Aquatic Sciences), Adelaide. SARDI Publication No. F2009/000406-1. SARDI Research Report Series No. 602. 143pp. Front Cover Images – Lake Brewster in the Lower Lachlan River catchment, Murray-Darling Basin during extended period of zero inflows, 2007. Murray cod (Maccullochella peelii peelii), olive perchlet (Ambassis agassizii) and golden perch (Macquaria ambigua) from the, lower Lachlan River near Lake Brewster, 2007 (all images - Dale McNeil). -

Assemblage Structure and Recruitment Patterns in a Dryland River Fish
Temporal changes in fish abundance in response to hydrological variability in a dryland floodplain river Author Balcombe, Stephen R, Arthington, Angela H Published 2009 Journal Title Marine and Freshwater Research DOI https://doi.org/10.1071/MF08118 Copyright Statement © 2009 CSIRO. This is the author-manuscript version of this paper. Reproduced in accordance with the copyright policy of the publisher. Please refer to the journal's website for access to the definitive, published version. Downloaded from http://hdl.handle.net/10072/25421 Link to published version http://www.publish.csiro.au/nid/126.htm Griffith Research Online https://research-repository.griffith.edu.au Temporal changes in fish abundance in response to hydrological variability in a dryland floodplain river Stephen R BalcombeA,B and Angela H ArthingtonA AAustralian Rivers Institute and eWater Cooperative Research Centre, Griffith University, Nathan, Queensland, 4111, Australia. B Corresponding author. Email: [email protected] Running Headline: Fish abundance patterns in a dryland river Abstract Riverine fish living in unpredictable flow environments, tend to be ecological generalists with traits that allow them to persist under highly variable and often harsh conditions associated with hydrological variation. Cooper Creek, an Australian dryland river, is characterised by extreme flow variability, especially in the magnitude, timing and duration of channel flows and floods, which, if they occur, do so mainly in summer. This study examined the influence of hydrological variability on fish assemblages and abundance in four waterholes in the Windorah reach of Cooper Creek over eight occasions between 2001 and 2004. Antecedent flows had marked influences on fish species richness and assemblage structure. -

Systematic Taxonomy and Biogeography of Widespread
Systematics and Biogeography of Three Widespread Australian Freshwater Fish Species. by Bernadette Mary Bostock B.Sc. (Hons) Submitted in fulfilment of the requirements for the degree of Doctor of Philosophy Deakin University February 2014 i ABSTRACT The variation within populations of three widespread and little studied Australian freshwater fish species was investigated using molecular genetic techniques. The three species that form the focus of this study are Leiopotherapon unicolor, Nematalosa erebi and Neosilurus hyrtlii, commonly recognised as the three most widespread Australian freshwater fish species, all are found in most of the major Australian drainage basins with habitats ranging from clear running water to near stagnant pools. This combination of a wide distribution and tolerance of a wide range of ecological conditions means that these species are ideally suited for use in investigating phylogenetic structure within and amongst Australian drainage basins. Furthermore, the combination of increasing aridity of the Australian continent and its diverse freshwater habitats is likely to promote population differentiation within freshwater species through the restriction of dispersal opportunities and localised adaptation. A combination of allozyme and mtDNA sequence data were employed to test the null hypothesis that Leiopotherapon unicolor represents a single widespread species. Conventional approaches to the delineation and identification of species and populations using allozyme data and a lineage-based approach using mitochondrial 16S rRNA sequences were employed. Apart from addressing the specific question of cryptic speciation versus high colonisation potential in widespread inland fishes, the unique status of L. unicolor as both Australia’s most widespread inland fish and most common desert fish also makes this a useful species to test the generality of current biogeographic hypotheses relating to the regionalisation of the Australian freshwater fish fauna. -

Western Queensland
Western Queensland - Gulf Plains, Northwest Highlands, Mitchell Grass Downs and Channel Country Bioregions Strategic Offset Investment Corridors Methodology Report April 2016 Prepared by: Strategic Environmental Programs/Conservation and Sustainability Services, Department of Environment and Heritage Protection © State of Queensland, 2016. The Queensland Government supports and encourages the dissemination and exchange of its information. The copyright in this publication is licensed under a Creative Commons Attribution 3.0 Australia (CC BY) licence. Under this licence you are free, without having to seek our permission, to use this publication in accordance with the licence terms. You must keep intact the copyright notice and attribute the State of Queensland as the source of the publication. For more information on this licence, visit http://creativecommons.org/licenses/by/3.0/au/deed.en Disclaimer This document has been prepared with all due diligence and care, based on the best available information at the time of publication. The department holds no responsibility for any errors or omissions within this document. Any decisions made by other parties based on this document are solely the responsibility of those parties. Information contained in this document is from a number of sources and, as such, does not necessarily represent government or departmental policy. If you need to access this document in a language other than English, please call the Translating and Interpreting Service (TIS National) on 131 450 and ask them to telephone Library Services on +61 7 3170 5470. This publication can be made available in an alternative format (e.g. large print or audiotape) on request for people with vision impairment; phone +61 7 3170 5470 or email <[email protected]>. -
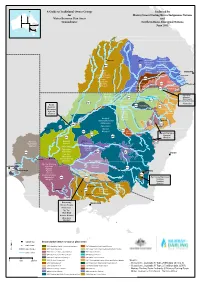
Guide to Traditional Owner Groups for WRP Areas Combined Maps
A Guide to Traditional Owner Groups Endorsed by for Murray Lower Darling Rivers Indigenous Nations Water Resource Plan Areas and Groundwater Northern Basin Aboriginal Nations June 2015 Nivelle River r e v i R e l a v i Nive River r e M M a Beechal Creek Langlo River r a n o a R i !( v Gunggari e Ward River Charleville r Roma Bigambul !( Guwamu/Kooma Barunggam Jarowair k e Bidjara GW22 e Kambuwal r GW21 «¬ C Euahlayi Mandandanji a «¬ Moola Creek l Gomeroi/Kamilaroi a l l Murrawarri Oa a k Bidjara Giabel ey Cre g ek n Wakka Wakka BRISBANE Budjiti u Githabul )" M !( Guwamu/Kooma Toowoomba k iver e nie R e oo Kings Creek Gunggari/Kungarri r M GW20 Hodgson Creek C Dalrymple Creek Kunja e !( ¬ n « i St George Mandandanji b e Bigambul Emu Creek N er Bigambul ir River GW19 Mardigan iv We Githabul R «¬ a e Goondiwindi Murr warri n Gomeroi/Kamilaroi !( Gomeroi/Kamilaroi n W lo a GW18 Kambuwal a B Mandandanji r r ¬ e « r g Rive o oa r R lg ive r i Barkindji u R e v C ie iv Bigambul e irr R r r n Bigambul B ive a Kamilleroi R rr GW13 Mehi River Githabul a a !( G Budjiti r N a ¬ w Kambuwal h « k GW15 y Euahlayi o Guwamu/Kooma d Gomeroi/Kamilaroi Paroo River B Barwon River «¬ ir GW13 Gomeroi/Kamilaroi Ri Bigambul Kambuwal ver Kwiambul Budjiti «¬ Kunja Githabul !( GW14 Kamilleroi Euahlayi Bourke Kwiambul «¬ GW17 Kambuwal Namo iver Narrabri ¬ i R « Murrawarri Maljangapa !( Gomeroi/Kamilaroi Ngemba Murrawarri Kwiambul Wailwan Ngarabal Ngarabal B Ngemba o g C a Wailwan Talyawalka Creek a n s Barkindji R !( t l Peel River M e i Wiradjuri v r Tamworth Gomeroi/Kamilaro -

Border Rivers and Moonie River Basins Healthy Waters
Healthy Waters Management Plan Queensland Border Rivers and Moonie River Basins Prepared to meet accreditation requirements under the Water Act 2007- Basin Plan 2012 Healthy Waters Management Plan: Queensland Border Rivers and Moonie River Basins Acknowledgement of the Traditional Owners of the Queensland Border Rivers and Moonie region The Department of Environment and Science (the department) would like to acknowledge and pay respect to the past and present Traditional Owners of the region and their Nations, and thank the representatives of the Aboriginal communities, including the Elders, who provided their knowledge of natural resource management throughout the consultation process. The department acknowledges that the Traditional Owners of the Queensland Border Rivers and Moonie basins have a deep cultural connection to their lands and waters. The department understands the need for recognition of Traditional Owner knowledge and cultural values in water quality planning. Prepared by: Department of Environment and Science. © State of Queensland, 2019. The Queensland Government supports and encourages the dissemination and exchange of its information. The copyright in this publication is licensed under a Creative Commons Attribution 3.0 Australia (CC BY) licence. Under this licence you are free, without having to seek our permission, to use this publication in accordance with the licence terms. You must keep intact the copyright notice and attribute the State of Queensland as the source of the publication. For more information on this licence, visit http://creativecommons.org/licenses/by/3.0/au/deed.en Disclaimer This document has been prepared with all due diligence and care, based on the best available information at the time of publication. -

The Diamantina and Warburton River System in South Australia
Natural Resources SA Arid Lands IMPROVING HABITAT CONDITION AND CONNECTIVITY IN SOUTH AUSTRALIA’S CHANNEL COUNTRY THE DIAMANTINA AND WARBURTON RIVER SYSTEM IN SOUTH AUSTRALIA Summary of technical findings June 2017 I Report to the South Australian Arid Lands Natural Resources Management Board, June 2017 Cover photograph: Koonchera Waterhole, South Australia All photos in this document were sourced from: the Technical Reports, Natural Resources SA Arid Lands, and others as specifically stated. The creators of this document acknowledge the significant direction and contribution provided by Henry Mancini. Document design and production by Cathryn Charnock Corporate Publishing Document can be referenced as: Mancini, H. (ed) 2017. Summary of technical findings: Improving habitat condition and connectivity in South Australia’s channel country - the Diamantina and Warburton river system in South Australia.Report by Natural Resources SA Arid Lands DEWNR, to the South Australian Arid Lands Natural Resources Management Board, Pt Augusta. Disclaimer: The South Australian Arid Lands Natural Resources Management Board, and its employees do not warrant or make any representation regarding the use, or results of use of the information contained herein as to its correctness, accuracy, reliability, currency or otherwise. The South Australian Arid Lands Natural Resources Management Board and its employees expressly disclaim all liability or responsibility to any person using the information or advice. While every reasonable effort has been made to verify the information in this report use of the information contained is at your sole risk. Natural Resources SA Arid Lands and the Australian Government recommend independent verification of the information before taking any action. © South Australian Arid Lands Natural Resources Management Board 2017 This work is copyright.