Soil Type in Uence Nutrient Availability, Microbial Metabolic Diversity
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nagar Nigam Rajnandgaon
Rajnandgaon is the quality city of Rajnandgaon District, in the state of Chhattisgarh, India. As of the 2011 census the population of the city was 163,122.[2] Rajnandgaon district came into existence on 26 January 1973, as a result of the division of Durg district.[3] Rajnandgaon City of Chhattisgarh Nickname(s): Nandgaon Rajnandgaon Location in Chhattisgarh, India Coordinates: 21.10°N 81.03°ECoordinates: 21.10°N 81.03°E Country India State Chhattisgarh District Rajnandgaon Government • Type Democratic • Body Nagar Nigam • Mayor Madhusudan yadav Elevation 307 m (1,007 ft) Population (2011)[1] • Total 163,122 • Rank 7th in State Time zone IST (UTC+5:30) PIN 491441 Telephone code 07744 Vehicle registration CG-08 Sex ratio 1023 ♂/♀ History[edit] Main article: Nandgaon State Originally known as Nandgram, Rajnandgaon State was ruled by Somvanshis, Kalachuris of Tripuri and Marathas.[4] The palaces in the town of Rajnandgaon reveal their own tale of the rulers, their society and culture, and the traditions of those times.[3] The city was ruled by a dynasty of Hindu caretakers (mahants), who bore the title Vaishnav and Gond rajas (chiefs). Succession was by adoption. Its foundation is traced to a religious celibate, who came from the Punjab towards the end of the 18th century. From the founder it passed through a succession of chosen disciples until 1879, when the British government recognized the ruler as an hereditary chief and it came to be known as princely state of Raj Nandgaon. Afterwards conferred upon his son the title of Raja Bahadur. The first ruler Ghasi Das Mahant, was recognized as a feudal chief by the British government in 1865 and was granted a sanad of adoption. -

Rajnandgaon Municipal Corporation
Rajnandgaon Municipal Corporation July 31, 2018 Summary of rated instruments Previous Rated Amount Current Rated Amount Instrument* Rating Action (Rs. crore) (Rs. crore) [ICRA]BB- (stable) ISSUER NOT Issuer rating NA NA COOPERATING; Withdrawn *Instrument details are provided in Annexure-1 Rating action ICRA has withdrawn the long-term issuer rating of [ICRA]BB-1 (pronounced ICRA double B minus)2 ISSUER NOT COOPERATING with a stable outlook assigned to the Rajnandgaon Municipal Corporation (RMC). Rationale The rating withdrawal follows the completion of the one-time rating exercise as per terms and conditions of Rating Agreement drawn with the State Urban Development Agency (SUDA), Government of Chhattisgarh. Analytical approach: For arriving at the ratings, ICRA has applied its rating methodologies as indicated below. Links to applicable criteria: Rating Methodology for Urban Local Bodies About the company: In the early 1970s, the urban local body (ULB) in Rajnandgaon was established as a municipality and, subsequently, it was upgraded to a municipal corporation in 1996. The Rajnandgaon Municipal Corporation (RMC) is governed by the Chhattisgarh Municipal Corporation Act, 1961 (Act), which is administered by the Urban Administration and Development Department (UADD), GoCG. According to Census 2011, the RMC, covering an area of 70.16 sq. km., serves a total population of 1.63 lakh. The key services extended by the corporation are construction and maintenance of roads and drains, water supply, solid waste management, street lights and amenities such as shopping stalls, community hall, playgrounds, parks/gardens etc. Status of non-cooperation with previous CRA: Not applicable Any other information: None 1 "With effect from Sep 1, 2017, ICRA has aligned the symbols and the definitions of ratings pertaining to the Issuer Rating Scale with that of the Long- Term Rating Scale. -

Omkaareswar Solvex Private Limited Detailed Description of the Secured Asset
OMKAARESWAR SOLVEX PRIVATE LIMITED DETAILED DESCRIPTION OF THE SECURED ASSET Lot. Plot Secured Asset Actual Area no No. demarcation 1. All that piece and parcel of land totally admeasuring 4.77 Acres along 4.77 Acres with structures situated thereon described as follows: All that piece and parcel of land bearing Kh. No. 128/7 admeasuring 3.00 acres, P H No 41/25 situated at Village Bhodiya, Gram Panchayat Bhotipar Khurd, District `Rajnandgaon and bounded as follows: East: Land of Devashish West: Road and Ghas Bhumi North: Land of Kamlesh South: Land of Lekharam together with factory sheds, buildings, structures constructed or to be constructed thereon. All that piece and parcel of land bearing Kh. No. 128/2 admeasuring 0.87acre and Kh.No.127/2 admeasuring 0.90 acres, P H No.41/25 situated at Village: Bhodiya, Gram Panchayat: Bhotipar Khurd, District: Rajnandgaon. Boundaries: Kh.No.128/2 East: Land of Ramprasad West: Land of Bainabai North: Land of bainabai South: Land of Sarvesh & others Boundaries: Kh.No.127/2 East: Nala West: Purchaser land North: Land of Govardhan South: Land of Yakub together with factory sheds, building, structures constructed or to be constructed thereon. 2. B-40 All that piece and parcel of diverted open residential plots bearing Kh. 1035 Sq. Ft No. 169/1, P H No.35/1, bearing No: B- 40, admeasuring 1,035 sq. ft. situated at Pandurang Nagar Housing Colony, Mahatma Gandhi Ward No.8, Tha. / Distt. Rajnandgaon, Chhattisgarh in the name of Mrs. Shefali Gupta, bounded by: North: Road South: Plot No. -

Bilaspur District of Madhya Prad~Sh
· B ," )tJ>,Jf~: __ GlAVb~trlA PRE F ACE Thi s ... epo.... t i; s the rt?s\.ll t. of the forest i nvento.ry carried out by the Central Zone of Forest Survey of India du... ing November 1985 to February 1986 1n Bilaspur district of Madhya Prad~sh. The report COVers the entire forest area of the district comprising three Forest Divisions viz. North Dilaspur(Pendra), Bilaspur and Korba. The inventory shows that out' of the recorded forest area of 7974.5 sq.km, about 92% area is under actual forest cover; ax of the area is either blank or under cultivation. Sal forests occupy 51.43% and MiMed forests occupy 44.95% of the forest. Salai fore~ts occur in 3.62% of the area. Sal is the dominant species in the district and contributes L~bout 31% to the totai growing stock. Other impC?rant species are saja and salai contributing nearly 11.9% of the total vol ume. The tot 1211 growi ng stoc~, in .the di ~tr i ct is about S6.5 million cubic metr~s, WhlCh works out at 82.95 cubic metres per hectar~ of th~ forest area. Bamboo occurs as an under storey in nearly 15X of the forest area. TQt~l yrowing stOC~( of bamboo is to the tune of 4.94 1 al:h tonn8s {ai r- dr-y wei ght) • This report was complIed by Shri S.C.Gupte, IFS, Joint Director, Forest Survey of India, Central Zone, Nagpur with the C:\s&istanc:e from Shri M.D.Singh, Senior Technical Assistant and Shri Anil Biala, Junior Technical Assistant. -

Sebuah Kajian Pustaka
International Journal of Research in Social Sciences Vol. 10 Issue 09, September 2020 ISSN: 2249-2496 Impact Factor: 7.081 Journal Homepage: http://www.ijmra.us, Email: [email protected] Double-Blind Peer Reviewed Refereed Open Access International Journal - Included in the International Serial Directories Indexed & Listed at: Ulrich's Periodicals Directory ©, U.S.A., Open J-Gate as well as in Cabell‟s Directories of Publishing Opportunities, U.S.A CONTRIBUTION OF FREEDOM FIGHTER THAKUR PYARELAL SINGH IN NATIONAL MOVEMENT VipinTirkey* Dr. Pradeep Shukla** (Research Scholar) (Professor) Department of History Department of History Guru GhasidasVishwavidyalaya Guru GhasidasVishwavidyalaya Bilaspur (C.G.) Bilaspur (C.G.) Abstract At the very beginning of 17th Century, the East India Company came into India during the reign of Queen Elizabeth of England for the trade purpose and the members of the company were to get more and more benefits through trading. But after the battle of Plassey in 1757, the company’s aim was for ruling not for trading. Slowly the East India Company became the ruling company first starting from Bengal and then later on their aim was to capture the throne of Mughal Baadshahin Delhi. After the revolt of 1857, the power was transferred from the company to British Crown. In this way, from 1857 to 1947, so called the nation India/Bharat was ruled over by the British Crown. Under the both rules Company and British Crown, they followed the policy of divide and rule, drained a lot of wealth from India to England, the people of India became poor to poorer, always paid heavy revenues to the rulers and so on. -

About Chhattisgarh
About Chhattisgarh Map Chhatisgarh state and districts (Stand 2007) At the time of separation from Madhya Pradesh, Chhattisgarh originally had 16 districts. Two new districts: Bijapur and Narayanpur were carved out on May 11, 2007[1] and nine new districts on Jan 1, 2012. The new districts have been created by carving out the existing districts to facilitate more targeted, focused and closer administration. These districts have been named Sukma, Kondagaon, Balod, Bemetara, Baloda Bazar, Gariaband, Mungeli, Surajpur and Balrampur[2] Contents [hide] 1 Background 2 Administrative history 3 Districts of Chhattisgarh 4 References 5 External links Background[edit] A district of an Indian state is an administrative geographical unit, headed by a district magistrate or a deputy commissioner, an officer belonging to the Indian Administrative Service. The district magistrate or the deputy commissioner is assisted by a number of officials belonging to different wings of the administrative services of the state. A superintendent of Police, an officer belonging to Indian Police Service is entrusted with the responsibility of maintaining law and order and related issues. Administrative history Before Indian independence, present-day Chhattisgarh state was divided between the Central Provinces and Berar, a province of British India, and a number of princely states in the north, south, and east, which were part of the Eastern States Agency. The British province encompassed the central portion of the state, and was made up of three districts, Raipur, Bilaspur, and Durg, which made up the Chhattisgarh Division of the Central Provinces. Durg District was created in 1906 out of the eastern portion of Raipur District. -

S.No Districts Covid Care Centre Hospital Category Total
S.No Districts Covid Care Centre Hospital Category Total Beds 1 Balod ITI Batera, Dondilohara Covid Care Center 100 2 Balod ITI Dhanora, Gurur Covid Care Center 100 3 Balod ITI Khallari, Gunderdehi Covid Care Center 60 4 Balod Sports Club Lal Maidan Dallirajhara Dondi Covid Care Center 100 5 Balod Livelyhood College Balod Covid Care Center 300 6 Balod PWD Colony Balod Covid Care Center 56 7 BALODA BAJAR DAV Khamhariya Baloda Bazar Covid Care Center 70 8 BALODA BAJAR ITI Simga Balodabazar Covid Care Center 112 9 BALODA BAJAR Livelyhood College Sankari Baloda Bazar Covid Care Center 161 10 BALODA BAJAR Pre Metric ST Hostal Kasdol Covid Care Center 50 11 Balrampur Polytecnic College Ramanunjganj Covid Care Center 250 12 Bastar Boys Hostal Bakawand Bastar Covid Care Center 200 13 Bastar Post Matric College Bastar Covid Care Center 250 14 Bemetara Lively hood college Bemetara Covid Care Center 130 15 Bemetara Agriculture College Covid Care Center 255 16 Bemetara Govt PG College Covid Care Center 100 17 Bemetara SRS Covid Care Center 25 18 Bijapur ITI Bairamgarh Bijapur Covid Care Center 100 19 Bijapur ITI rudraram Covid Care Center 100 20 Bilaspur Central Hospital railway Covid Care Center 75 21 Bilaspur Chitrakoot hostel bilaspur Covid Care Center 200 22 Bilaspur Community Hospital CH Bharni Covid Care Center 50 23 Bilaspur Mental Hospital Sendri Bilaspur Covid Care Center 10 24 Bilaspur NTPC seepat Covid Care Center 10 25 Bilaspur Prayas Hostel Bilaspur Covid Care Center 400 26 Dantewada Covid Care Center Eklavya Parisar Jawanga Covid Care -

LIST of FILED from 2021-02-22 to 2021-02-26 Sr.No IEM NO & DATE
LIST OF FILED From 2021-02-22 To 2021-02-26 Sr.No IEM NO & DATE Name & Address Of The District & State Item Of Manufacture Party 1 231/SIA/IMO/2021 INDIASKILLPEDIA KAMRUP ASSAM Manufacture of ploughs, manure 22/02/2021 FOUNDATION Plot No 14 spreaders, seeders, harrows and similar Uphill Villa ASSAM agricultural machinery for soil preparation, planting KAMRUP 781020 or fertilizing, harvesting or threshing machinery 2 232/SIA/IMO/2021 THE KARNAL CO-OP KARNAL HARYANA ELECTRIC POWER GENERATION USING OTHER NON 22/02/2021 SUGAR MILLS LTD 3KM CONVENTIONAL SOURCES STONE MEERUT ROAD HARYANA KARNAL 132001 3 233/SIA/IMO/2021 KINGS GAS PRIVATE GREATER LNG GASES 22/02/2021 LIMITED A-101 MUMBAI(MUMBAI) SATSANG-II CHS LTD MAHARASHTRA MAHARASHTRA THANE 401107 Sr.No IEM NO & DATE Name & Address Of The District & State Item Of Manufacture Party 4 234/SIA/IMO/2021 RICHA GLOBAL GURGAON READYMADE GARMENTS 22/02/2021 EXPORTS PRIVATE (GURUGRAM) HARYANA LIMITED A-41, DELHI DELHI 110064 5 235/SIA/IMO/2021 ULTRA ENGINEERS PUNE MAHARASHTRA GAS ENGINE PARTS 22/02/2021 PLOT NO-58 SECTOR NO-10 MAHARASHTRA PUNE 411026 6 236/SIA/IMO/2021 Ginza Industries Limited A PALGHAR Narrow Knitted Fabric with or without elastomeric yarn 22/02/2021 -501 & 502 Lotus Corporate MAHARASHTRA Park Jay Coach Lane MAHARASHTRA GREATER MUMBAI(MUMBAI) 400063 7 237/SIA/IMO/2021 ADISANKARA SPINNING DINDIGUL MANNAR MANUFACTURING OF GARMENTS 22/02/2021 MILLS PRIVATE LIMITED THIRUMALAI TAMIL OLD NO 86 NEW NO 207 NADU MANGALAM ROAD TAMIL NADU TIRUPUR 641604 Sr.No IEM -

District Fact Sheet Rajnandgaon Chhattisgarh
Ministry of Health and Family Welfare National Family Health Survey - 4 2015 -16 District Fact Sheet Rajnandgaon Chhattisgarh International Institute for Population Sciences (Deemed University) Mumbai 1 Introduction The National Family Health Survey 2015-16 (NFHS-4), the fourth in the NFHS series, provides information on population, health and nutrition for India and each State / Union territory. NFHS-4, for the first time, provides district-level estimates for many important indicators. The contents of previous rounds of NFHS are generally retained and additional components are added from one round to another. In this round, information on malaria prevention, migration in the context of HIV, abortion, violence during pregnancy etc. have been added. The scope of clinical, anthropometric, and biochemical testing (CAB) or Biomarker component has been expanded to include measurement of blood pressure and blood glucose levels. NFHS-4 sample has been designed to provide district and higher level estimates of various indicators covered in the survey. However, estimates of indicators of sexual behaviour, husband’s background and woman’s work, HIV/AIDS knowledge, attitudes and behaviour, and, domestic violence will be available at State and national level only. As in the earlier rounds, the Ministry of Health and Family Welfare, Government of India designated International Institute for Population Sciences, Mumbai as the nodal agency to conduct NFHS-4. The main objective of each successive round of the NFHS has been to provide essential data on health and family welfare and emerging issues in this area. NFHS-4 data will be useful in setting benchmarks and examining the progress in health sector the country has made over time. -
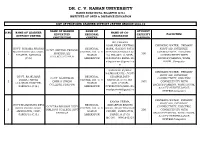
Click Here to See List of Proposed Study Center
DR. C. V. RAMAN UNIVERSITY KARGI ROAD KOTA, BILASPUR (C.G.) INSTITUTE OF OPEN & DISTANCE EDUCATION LIST OF PROPOSED LEARNER SUPPORT CENTRE SESSION 2018-19 NAME OF HIGHER NAME OF STUDENT S.NO NAME OF LEARNER NAME OF CO- EDUCATUIN REGIONAL CAPECITY FACILITIES . SUPPORT CENTRE ORDINATOR INSTITUTION CENTRE (APPROX) MD.PARWEZ ALAM,NEAR CENTRAL DRINKING WATER, PRIMARY GOVT. SHYAMA PRASAD REGIONAL BANK, GAURAV PATH FIRST AID, INTERNET, GOVT. SHYAMA PRASAD MUKHARJEE COLLEGE, CENTRE, DR. C. V. SITAPUR,SARGUJA,(C. CONNECTIVITY, ELECTRIC 1 MUKHARJEE 300 SITAPUR, SARGUJA RAMAN G.) PIN-497111,MOB- CONNECTIVITY WITH COLLEGE,SITAPUR (C.G.) AMBIKAPUR 9424261584 EMAIL ID- BACKUP,PARKING, WASH [email protected] ROOM.,Computer m SANDEEP KUMAR DRINKING WATER, PRIMARY RAJWADE,VILL-,POST- FIRST AID, INTERNET, GOVT. RAJKUMAR REGIONAL UDAIPUR,DIST- GOVT. RAJKUMAR CONNECTIVITY, ELECTRIC DHIRAJ SINGH CENTRE, DR. C. V. SARGUJA,(C.G.),PIN- 2 DHIRAJ SINGH 1000 CONNECTIVITY WITH COLLEGE,UDAIPUR, RAMAN 497117,MOB- COLLEGE,UDAIPUR BACKUP,PARKING, WASH ROOM, SARGUJA (C.G.) AMBIKAPUR 07909870934,MAIL ID- & CCTV SURVEILLANCE [email protected] SYSTEM,Computer m DRINKING WATER, PRIMARY KAMNA VERMA, FIRST AID, INTERNET, GOVT.RAJMOHANI DEVI REGIONAL KEDARPUR BHATHI GOVT.RAJMOHANI DEVI CONNECTIVITY, ELECTRIC GIRLS PG COLLEGE, CENTRE, DR. C. V. ROAD, NEAR TRICHON 3 GIRLS PG COLLEGE DIST- 200 CONNECTIVITY WITH AMBIKAPUR, DIST- RAMAN CHOWK, AMBIKAPUR, SARGUJA BACKUP,PARKING, WASH ROOM, SARGUJA (C.G.) AMBIKAPUR SARGUJA (C.G.) & CCTV SURVEILLANCE MO.NO. - 9981642327 SYSTEM,Computer VARSHA DRINKING WATER, PRIMARY KASHYAP,Takiya road, FIRST AID, INTERNET, REGIONAL kenabandh ambikapur, RAJEEV GANDHI GOVT. CONNECTIVITY, ELECTRIC RAJEEV GANDHI GOVT. CENTRE, DR. C. V. Distt. Surguja 4 COLLEGE AMBIKAPUR 600 CONNECTIVITY WITH COLLEGE,AMBIKAPUR RAMAN (C.G.),Cell: +91 (C.G.) BACKUP,PARKING, WASH ROOM, AMBIKAPUR 9424255074 & CCTV SURVEILLANCE Email : SYSTEM,Computer [email protected] Rinu Jain Manjusha Academy Namna Kala , Ring DRINKING WATER, PRIMARY Road Ambikapur , FIRST AID, INTERNET, REGIONAL K.R. -

2017 Saturday & Sunday, 15Th & 16Th July 2017 at Hotel Conard ,Pune
Feed & Feed Ingredients Conclave – 2017 Saturday & Sunday, 15th & 16th July 2017 at Hotel Conard ,Pune Speakers / Special Invitees List Status Reg.No. Mr. Delegate Name Company Name City Organisor I-001 Mr. Atul Chaturvedi Adani Group Ahmedabad Organisor I-002 Mr. B. Soundararajan Suguna Foods Ltd. Coimbatore Organisor I-003 Mr. Sandeep Bajoria Bajoria Fats & Proteins Ltd. Mumbai Guest of Gujarat Cooperative Milk Marketing Honour I-004 Mr. R. S. Sodhi Federation Anand Guest of Dept. of Animal Husbandray, Dairying & Honour I-005 Mr. O. P. Chaudhary Fisheries N. Delhi Hon'ble Guest I-006 Dr. D.Y. Patil Food & Civil Supplies and Consumer Hon'ble Guest I-007 Mr. Girish Bapat Protection Mumbai Organisor I-008 Mr. Pravin Lunkad Pranav Agro Industries Ltd. Pune Speaker I-009 Mr. Vijay Sardana Agri Business Delhi Speaker I-010 Dr. Ajit Ranade Shirwal Veterinary College Pune Speaker I-011 Mr. Ravi Kumar Yellanki Vaisakhi Bio Marine Pvt. Ltd. Visakahpatanam Speaker I-012 Mr. Prasad P. Yes Bank Ltd. Mumbai Speaker I-013 Dr. Pawan Kumar USSEC Speaker I-014 Dr. D. Srinivas Kumar Krishn Vignan Kendra Guntur Speaker I-015 Mr. Aurobinda Prasad Kotak Commodity Services Ltd. Mumbai Speaker I-016 Dr. Vijay Makhija DSM Nutritonal Products Thane Speaker I-017 Dr. Dinesh Bhosale A.B. Vista South Asia Pune Raghavan Sampath Speaker I-018 Mr. Kumar SMARTA Biz Speaker I-019 Mr. Shiva Mudgil Rabo India Finance Ltd. Mumbai Speaker I-020 Mr. Nagraj Meda Transgraph Consulting Pvt. Ltd. Hyderabad Speaker I-021 Ms. Manisha Gupta CNBC TV 18 Mumbai Speaker I-022 Mr. -

Salhewara Block, Dist. Rajnandgaon
PROPOSAL FOR RECONNAISSANCE SURVEY FOR IRON ORE IN SALHEWARA AREA OF DISTRICT RAJNANDGAON, CHHATTISGARH (G-4) (THROUGH NMET FUND) GOVERNMENT OF CHHATTISGARH MINERAL RESOURCES DEPARTMENT 1 PROPOSAL FOR RECONNAISSANCE SURVEY FOR IRON ORE IN SALHEWARA AREA (G-4) OF DISTRICT RAJNANDGAON, CHHATTISGARH EXPLORATION -07 SUMMARY OF THE BLOCK: 1 Title of project Proposal for Reconnaissance Survey for Iron Ore in Salhewara area (G-4) of district Rajnandgaon, Chhattisgarh 2 Mineral/minerals to be explored Iron ore 3 Objective Demarcation and Assessment of Iron Ore deposits 4 Level of proposed Exploration (G4/G3/G2/G1) G-4 5 State Chhattisgarh District Rajnandgaon Block/Area Salewara 6 Toposheet No. & 64 C/14 Forest comportment No. 153,154 7 Total area and 100 Sq. km. for mapping Coordinates of the area/block (1:12,500 scale) (Latitude/Longitude) A. 80º46’35.12” - 21º39’13.13” B. 80º53’12.79” - 21º39’13.13” C. 80º53’12.79” - 21º33’04.97” D. 80º46’35.12” - 21º33’04.97” 5.2025 Sq.km. (thrust area) 1 80.81822 21.61869 2 8081330 21.62287 3 80.81845 21.62477 4 80.82423 21.62059 5 80.82760 21.61795 6 80.83082 21.61770 7 80.83374 21.61681 8 80.83472 21.61274 9 80.83909 21.60976 10 8083984 21.60769 11 80.83899 21.60493 12 80.84014 21.60310 13 80.84290 21.60087 14 80.84591 21.59943 15 80.85089 21.59993 16 80.84297 21.59726 17 80.83732 21.59461 18 80.83331 21.58978 19 80.82967 21.58474 20 80.82739 21.58783 21 80.82985 21.58904 22 80.82953 21.59713 23 80.82514 21.60336 24 80.82891 21.60545 25 80.82473 21.60722 26 80.82454 21.61227 NMET/Iron/Salhewara/Nagpur/21.05.2018 2 27 80.82289 21.61353 28 80.82037 21.61467 29 80.82067 21.61662 30 80.81822 21.61869 8 Background information a.